Seaweed is an aquaculture commodity that has a high economic value. Production of seaweed Gracilaria was estimated to increase concurrent with the increasing demand of seaweed for food industry, pharmacy and cosmetics. Gracilaria has been cultivated worldwide. Gracilaria verrucosa and G. gigas are commonly cultured in brackish water ponds in Indonesia such as in South Sulawesi (Jeneponto, Takalar, Sinjai, Bulukumba, Wajo, Palopo, Bone and Maros Regencies), North Coast of Java (Serang, Tangerang, Bekasi, Karawang, Brebes, Pemalang, Tuban and Lamongan Regencies) and West Lombok (DKP, 2005).
The development of Gracilaria cultivation in ponds, in addition to increase the productivity and income of breeding farms, it is also expected to improve the quality of pond environment.. By cultivating the Gracilaria sp in brackish-water ponds the production of oxygen is increasing due to the increasing of the photosynthesis and the decreasing of the nitrite due to the nitrification process. In the other hand the production of the brackish-water ponds (milkfish and shrimp) can be increased. Seaweed is one of alternativesthat can be used to improve the pond water environment, because it has ability to absorb nutrients, so that can be used as a biofilter, bioakumulator as well as biomonitoring pollution that occurs in aquaculture pond waters (Komarawidjaja, 2005). Thus, the causative agent of ice-ice disease can affect the sea weed as a host of bacteria.
One of the constraints experienced in the process of cultivation of seaweed isdisease. The attack of ice-ice diseaseincreased in line with pathogenic bacterial infection of the seaweed thalli. The condition is caused by the increasing of pathogenic bacteria activity in secrete virulence factors. The development of thepathogenic bacteriaactivity on seaweed thalli result in white patches on the thallus and gradually become brittle and eventually thallus fracture, resulting in decreased production of seaweed range of 70-100% (Vairappan et al. 2008).
The results of the identification onthe seaweed Kappaphycus alvarezii infected byice-ice disease is caused by apathogenic bacterianamely;Vibrio alginolyticus, Pseudomonas cepacia, Flavobacterium meningosepticum, Pseudomonas diminuta and Plesiomonas shigelloides (Aris, 2011). Types of bacteria that cause ice-ice disease arePseudomonas nigricaciens, Pseudomonas fluorescens, Vibrio granii, Bacilllus cercus and Vibrio agarliquefaciens Yulianto (2002).
The mmanagement ofgood seaweed cultivation and free of ice-ice disease arethemeans in improving production technology of G.verrucosa. The disease needs to be studied through isolation, identification and control of pathogenic bacteria in G. Verrucosa. .
1 Materials and Methods
1.1 Place of Experimental Seaweed
Seaweed Gracilaria verrucosa was taken from brackishwater pond at Takalar Regency, South Sulawesi Province, Indonesia. Ice-ice infected thalli of G. verrucosa were washed with sterile water and placed into sterile plastic bags. Samples were then placed in the cool box and transported to laboratory. Isolation and biochemical characterization of bacteria were carried out in Marine Microbiology Laboratory, Faculty of Marine Sciences and Fisheries, Microbiology and Immunology Laboratory, Faculty of Medicine, Hasanuddin University, and Institute of Brackishwater Aquaculture Development, Maros.
1.2 Bacterial Isolation and Identification
Isolation and identification of bacteria was conducted from ice-ice infected branches of seaweed Gracilaria verrucosa.
One gram of ice-ice infected branches of Gracilaria verrucosa was homogenized in 9 ml sterile NaCl 0.9% and dilution series up to 10-6 were made up. One milliliter of aliquot from each dilution series was spread-plated onto tryptic soy agar (TSA) and thiosulphatecitrate bile sucrose (TCBS) agar plates and then incubated at 30°C for 24 h. For purification and identification, differentially isolated colonies were randomly picked and streaked onto the TSA medium. Identification of bacteria was conducted based on the morphology of colonies and biochemical characterizations.
1.3 Biochemical Test
Identification of bacteria was done through the biochemical test to find out the behavior or characteristic of the bacteria according to Capuccino and Sherman, (1987), that included the Gram staining, OF, oxidase test, catalase test, SIM test (indole, motile, gas, H2S), TSIA test (butt, slant, H2S, gas), MR, VP, King A and King B. The nature or characteristic of bacteria by using medium TCBS (Thoisulphate Citrate Bile Salt Sucrose Agar).
Characterization is one of the activities carried out to observe the results of bacterial isolation (isolates). Characterization activities can be carried out based on the nature of cytology (cell form, movement or motility, Gram nature), morphology, and physiological nature. Test morphological naturesincludethe natures ofcolonies, such as size, shape, color and edges, while the physiological naturesuch testing starch hydrolysis test, hydrolysis of fat, protein hydrolysis and catalase test. Test of Gram staining was conducted to group bacteria into 2 big groups according to its cell wall structure namely Gram-positive and Gram-negative bacteria. The staining was important stage in the bacteria characterization and identification (Lay, 1994). The catalase test was conducted to find out whether a bacteria has catalase enzyme in which the enzyme oxidized H2O2 (Cappuccino and Sherman, 1987).
2 Results
2.1 Bacterial Isolation and Identification
The results of the observation on the thalli showed that the gross signs of the thalli infected with ice-ice disease were initiated with translucent appearance of the proximal, median or distal end of the thalli leading to whitening of the whole thalli (Figure 1). Santoso (2008) reported that ice ice infection in seaweed attacked the base of thallus, stem and the tip of thallus that caused the tissue become white and soft.
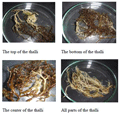
Figure 1 Gross signs of the thalli infected with ice-ice disease
|
There were eight bacterial isolates with different colonial morphologies found in the infected thalli of the seaweed (Table 1).
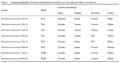
Table 1 Colonial morphology of bacteria isolated from Gracilaria verucosa infected with ice-ice diseases.
|
Bacterial isolate was successfully isolated from the seaweed G. verrucosa infected by ice-ice. Morphology of bacteria colony was almost entirely circular, except at Gv.1 isolates. Almost all isolates were entire colony margins, except Gv.2, Gv.3 and Gv.4 which were serrate, elevation is generally convex, except Gv.4 and Gv.5 are flat. The color of the colony on Gv.1 and Gv.5 isolates where white, the isolates Gv.2 was yellow, Gv.4, Gv.7 and Gv.8, were cream, and orange on Gv.3 and Gv.6 isolates. These data were appropriate with funding of Cappucino and Sherman (1987), that bacterial colonies shape in generalcircular, irreguler, filamentous, rhizoid, the elevation wasraised, convex, flat, umbonate, crateriform. Margin shapes entire, undulute, filiform, curled and lobate.
2.2 Characteristic of Bacteria
The result of gram staining of ice-ice bacteria isolates in G. verrucosa was obtained that bacteria isolates Gv.1, Gv.2, Gv.6, Gv.7, and Gv.8 belonged to gram-negative. While isolates Gv.3, Gv.4 and Gv.5 belonged to Gram-positive. The result of catalase test showed that isolates Gv.1, Gv.2, Gv.3 and Gv.4 showed positive catalase reaction, while isolates Gv.5 and Gv.6 showed negative reaction (Table 2).
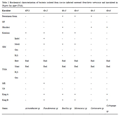
Table 2 Biochemical characterization of bacteria isolated from ice-ice infected seaweed Gracilaria verrucosa and inoculated in Tryptic Soy Agar (TSA).
|
The SIM test (gas, H2S) and TSIA (gas, H2S) showed that all isolates were negative. It was caused by the formation of H2S marked by the black sign in the medium and the formation of gas marked by the rupture of medium at the lower end of the glass tube.
Biochemical characterization of the isolates showed the presence of Acinetobacter sp.at Gv.1, Pseudomonas sp. at Gv.2, Bacillus sp. at Gv.3, Micrococcus sp. at Gv.4, Corinoverm sp. at Gv.5, Cytophaga sp. at Gv.6 (Table 2), Vibrio mimicusatGv.7 and Vibrio Campbelii at Gv.8 (Table 3) in the ice-ice disease infected thalli.

Table 3 Biochemical characterization of bacteria isolated from ice-ice infected seaweed G.verrucosa and inoculated in Thiosulphate Citrate Bile Sucrose Agar(TCBS).
|
3 Discussion
3.1 Acinetobacter sp.
The results of staining obtainedshow that Acinetobacter sp. included in the gram-negative marked in red on the bacterial cell. The results of characteristic (Table2) showed that surface of the colonyisshiny, not motile, oxidase negative, does not produce pigment, catalase positive, and white.
3.2 Pseudomonas sp.
Pseudomonas sp. is a rod Gram negative bacteria, motile (some have polar flagellum) and catalase positive. Generally, colonial bacteria are yellow, facultative anaerobic or aerobic, can grow at 4–43 °C. Most species have an optimum temperature around 30 °C (Buchanan and Gibbons, 1974).
Pseudomonas generally can be found in the soil, freshwater and seawater (Buchanan and Gibbons, 1974). These bacteria frequently isolated from plant and animal surfaces. Some members of these genera considered as a true pathogen in plants (Todar, 2013). Pseudomonas grows in injured tissues and produces toxins leading to the death of surrounding cells (Moorman, 2013). Some species such as Pseudomonas. aeruginosa, are opportunistic pathogen releasing extracellular protease that can infect host tissues (Buell et al. 2003).
Amiluddin (2007) identified five bacteria causing ice-ice disease on K. alvarezii including Bacillus cereus, Vibrio granii, V. liquefaciens, P. nigricaciens, and P. fluorescens. Darmayanti et al. (2003) also isolated Pseudomonas sp., Vibrio sp. and Aeromonas sp. in healthy and infected thalli of cultured K. alvarezii in Pari Island. In addition, Pseudomonas sp. was found on cultivated seaweed in coastal water in Takalar Regency, South Sulawesi, Indonesia
3.3 Bacillus sp
Bacillus is a Gram negative bacteria, rod, motile (some non-motile), catalase positive and oxidase positive. Bacillus may form endospore, aerobic and facultative anaerobic (Feliatra, 2004). Bacillus can be isolated from soil and water including seawater. These bacteria can be found as a pathogen, opportunistic saprophytes. Some species produced extracellular enzymes that have ability to hydrolyze proteins and polysaccharides (Pelczar et al. 1975).
Bacillus sp. produced spores that are heat resistant and have ability to degrade xylan and carbohydrates (Cowan and Steel, 1973). Bacillus sp. has ability to grow at temperature more than 50 °C and less than 5 °C, at high concentration of salt (> 10%), resistant to pasteurization, produce spores and has higher proteolytic activities than other microbes.Bacillus sp. resistant to environmental conditions like resistance of heat, acid and extreme salt content. Kinds of Bacillus produce extracellular enzymes which can hydrolyze protein and polysaccharide complex.
3.4 Micrococcus sp
Micrococcus is a Gram positive bacteria, sphere (cocci) with a size of 0.5–3.0 µm in diameter. It is aerobic and anaerobic and has an optimum temperature of 25–37℃ to grow. These bacteria can be found as a pathogen, opportunistic saprophytes and commensal organism. The role of this bacteria in the ground is asdecomposers to decomposeorganic material into its composing constituent that can be used directly by plants.
3.5 Cytophaga sp
Cytophaga is a rod Gram negative bacteria. Some member within this group can cause diseases in fish. These bacteria can utilize cellulose and agar/chitin. This microbe can produce cell membranes containing endogluconases and periplasmic exogluconases that have activity in degrading cellulose. These bacteria can attack cellulose, but they cannot produce cellulose. This enzyme can only bind to the cell envelope , mix with mucus and is released during movement. This bacterium inhibits soil and water. The colony of these bacteria is yellow or orange and can be grown in the medium containing cellulose. Largo et al. (1995), who isolated bacteria in Kappaphycus obtained 10 bacteria strains Cytophaga sp. (Cytophaga- Flavobactroium).
3.6 Vibriosp
Vibrio have a characteristic of curved rod Gram negative bacteria with a size of 1 – 3 × 0.4 – 0.6 µm, non-forming spore and capsule, and motile with a polar flagella. This saprophytic microbe becomes pathogenic when the environment quality depleted. According to Kabata (1988), Vibrio is a pathogenic bacteria that commonly cause problems to the aquatic organisms. Largo et al. (1995) isolate Vibrio sp from the infected K. alvarezii. Recently, a study on ice-ice infected K.alvareziirevealed the presence of V. alginolyticus, P. cepacia, P. diminuta, Plesiomonas shigelloides and Flavobacterium meningosepticum in the infected thalli (Aris, 2011). Furthermore, Bockemihi et al. (1986), Chowdury et al. (1989) in Eaves (1994) reported that Vibrio mimicus was found in brackish water and fresh water.
Largo et al. (1999) explained the mechanism of the Vibrio infection in seaweed thallus was when the seaweed under stress. The Vibrio would breed in the cell wall by using polysaccharide as the media or carbon source. Furthermore, Lin in Yulianto (2009) explained that after 2-3 days, Vibrio entered the tissue until the medullary seam by pumping the caraginase enzyme then caused the thallus became pale/white and its tissue was soft and easily broken.
Acknowledgement
The authors thank the Ministry of Education and Culture of the Republic of Indonesia for provided the funding research , Thank also our fiends (Huyyirnah, Rusaini, Ph.D. Cand.) for their assistance in the provide.
Amiluddin N. M., 2007, Study of Growth and Carragenan Content of Seaweed Kappaphycus alvarezii (Lin.,1758), Affected by Ice ice in the Water of Puri island, Seribu Islands, Thesis for M.S, Graduate Scholl of Bogor Agricultural University, Bogor, pp.1-78
Aris M, 2011, Identification, Pathogenicity of Bacteria and the Use of Gene 16S rRNA for Ice-ice Detection on Seaweed Aquaculture , Kappaphycus alvarezii (Linn., 1758), Thesis for M.S, Graduate School of Bogor Agricultural University (IPB), Bogor, pp.1-127
Austin B., and Austin D.A., (eds). 1993, Bacterial fish pathogen disease in farmed and will fish, Second Edition, Ellis Horwood Limited. Department of Biological Sciences, Heriot-Watt University, England, pp.553
Buchanan R.E., and Gibbons N.E., 1974, Bergey’s Manual of Determinative Bacteriology, 8th ed. USA, The Williams & Wilkins Co, Inc, pp.1272
Buell C.R., Joardar V., and Lindeberg M., 2003, The Complete Genome Sequence of the Arabidopsis and Tomato Pathogen Pseudomonas syringae pv. Tomato DC3000, Proc. Natl. Acad. Sci. USA 100:10181-10186
http://dx.doi.org/10.1073/pnas.1731982100
Cappuccino J.G., and Sherman N., 1987, Microbiology a Laboratory Manual (7th ed.). San Francisco, The Benjamin/Cummings Publishing Company, Inc, pp.458
Cowan S.T., and Steels D., 1973, Manual for identification of medical bacteria, Second Edition, Cambridge University Press, London, pp.352
Darmayanti D. 2001, Fusarium Function, http://sciweb.nybg.org/science2/hcol/fusarium3.asp .(Online).
Directorate General of Aquaculture, 2005, Fisheries Revitali- zation. Department of Marine and Fisheries, Jakarta
Eaves L.E, and Katterer P.J., 1994, Mortalities in Red Claw Crayfish Cherax quadricarinatus Associated with Systematic Vibrio mimicus Infection, Diseases of Aquatic Organisms, 19: 233-237
Feliatra I.E., Efendi I., and Suryadi E., 2004, Isolation and Identification of Probiotic Bacteria Tiger Grouper (Ephinephelusfuscogutatus) in the Effort of Fish Feed Efficiency, Indonesian Nature Journal, 6(2): 75-80
Kabata Z,. 1988, Parasites and diseases of fish culture in the Tropics, Taylor and Francis. London, pp.318
Komarawidjaja W., 2005, Seaweed Gracilaria sp. as Fitoremedian Pond Aquaculture Aquatic Organic Materials, Jur.Tekn. Ling. P3TL (Center for Environmental Assessment and Application of the Technology), Jakarta, 6(2): 410-415
Largo D.B., Fukami K., and Nishijima T., 1995, Occasional Pathogenic Bacteria Promoting Ice-ice Disease in the Carrageenan-producing Red Algae Kappaphycus alvarezii (Linn., 1758) and Euchema denticulatum (Solieriaceae, Gigartinales, Rhodophyta), Journal of Applied Phycology, 7: 545-554
Largo D.B., Fukami K., and Nishijima T., 1999, Time-dependent attachment mechanism of bacterial pathogen during ice–ice infection in Kappaphycus alvarezii (Linn., 1758) (Gigartinales, Rhodophyta), Journal of Applied Phycology, 11: 129-136
Lay W., Bibiana., 1994, Microbial Analysis in a Laboratory, PT Raja Grafindo Persada, Jakarta, pp.168
Nurjanna, M., 2008, Bacterial Identification Isolated from Seaweed with Ice ice Disease, Bul. Tech. Lit. Aquaculture, 7(1): 79-81 (in Indonesian)
Pelczar M.J., Chan E.C.S., 2005, Basics of microbiology, 1st and 2ndVolume. Hadioetomo R.S, Imas T, Tjitrosomo S.S, Angka S.L, translator, Jakarta: Universityof Indonesia Press, Translation from: Element of Microbiology, pp.997
Santoso L., and Nugraha Y.T., 2008, The Control of Ice ice Disease to Improve Seaweed Production in Indonesia. Science and Technology Journal of Fisheries. 3(2): 37-43 (in Indonesian)
Vairappan C. S., Chung C.S., Hurtado A.Q., Soya F.E., Bleicher-Lhonneur G., Critchley A., 2008, Distribution and Symptoms of Epiphyte Infection in Major Carrageenophyte-Producing Farms. J. Appl. Phycol. 20: 477–483
Yulianto, K and Mira S., 2009, Macro Algae Cultivation of Kappaphycus alvarezii (Linn., 1758) Vertically and Symptoms of Ice ice disease in the Water of Pari Island. Pulau Pari, Oceanology and Limnology Journal, 35(3): 323-332 (in Indonesian)

 Author
Author  Correspondence author
Correspondence author






