Research Report
Ecto-parasites Infestation of Nile Tilapia (Oreochromis niloticus) in Concrete Ponds in Tamale, Ghana 
2. Council for Scientific and Industrial Research-Water Research Institute, Fishery Division, P.O. Box TL 695, Tamale, Ghana
 Author
Author  Correspondence author
Correspondence author
International Journal of Aquaculture, 2015, Vol. 5, No. 4 doi: 10.5376/ija.2015.05.0004
Received: 12 Feb., 2015 Accepted: 15 Mar., 2015 Published: 30 Apr., 2015
Baidoo et al., 2015, Ecto-parasites Infestation of Nile Tilapia (Oreochromis niloticus) in Concrete Ponds in Tamale, Ghana, International Journal of Aquaculture, Vol.5, No.3 1-5 (doi: 10.5376/ija.2015.05.0004)
The demand of concrete pond culture in the Northern Ghana is increasing. The safety and health of Nile tilapia produced in concrete ponds is of public concern. The study was to investigate the prevalence and mean intensities of ecto-parasites on the gills, skin mucus and the fin of Nile tilapia (Oreochromis niloticus) in some concrete ponds in Tamale, Northern Ghana. Two hundred and forty (240) randomly sampled specimens of comprising fingerlings, juveniles and adult fish of Nile Tilapia were examined using microscope and hand-held lens from November, 2013 to April, 2014 for ecto-parasites. Thirty five (35) of the specimens examined were infested with ecto-parasites representing 15% prevalence during the study period. The prevalence rate encountered was Ichthyophthirius multifilis (60%) ≥; Trichodina sp (23%) ≥; monogeneans (17%). Out of 155 fingerlings examined, 14.8% were infested while 85 adult fish examined had 14.1% infested with ecto-parasites. The mean intensity of Trichodina spp ranged from 0 to 2.5. Monogeneas and Ichthyophthirius multifilis mean intensity ranged from 0 to 1.7. Physico-chemical characteristics of the pond were suitable for culture. The mean temperature was 28.48 oC ± 2.31, the mean value for dissolved oxygen was 4.32 mg/l ± 0.38, the mean pH was 6.17 ± 0.23 and mean nitrate was 30.60 ppm ± 1.0286. The low prevalence and intensities of ecto-parasites recorded indicates that with good water quality, ecto-parasites may not pose a threat to O. niloticus production in concrete pond.
Fish form a major component in the diet of the protein- deficient majority of the rural poor in the northern Ghana FAO (2005). Recent advances in fish farming, especially with some African Cichlids, have alleviated hunger in many parts of the world (FAO, 2005; Aquaculture production, 2004). Among numerous factors that affect the production of Cichlids particularly Nile tilapia, one important factor which is often overlooked is parasitic infections and diseases. Tilapia is becoming the most cultured fish native to Africa (Agbeko et al., 2014). Fish as well as other vertebrates are either intermediate host or definitive host to many parasite species. It is therefore important to identify parasites that affect tilapia production in the different stage of growth, their prevalence and intensity in the fish populations and communities and also establish their distribution in a defined geographical region.
The high risk of fish disease transmission and parasite infestation among Tilapia has increased the level of uncertainty which farm managers have to contend with to develop the industry (Pozio and Rosa, 2005). The majority of the disease-causing pathogens are protozoans, monogenetic trematodes and parasitic crustaceans (e.g. salmon lice in farmed Atlantic salmon in Norway and Ireland), most of which have direct life cycles and reproduce rapidly under unfavourable pond conditions (Al-Rasheid et al., 2000; Basson and As, 1994; Van As and Basson, 1987).
Parasitological knowledge has been useful in the development of the aquaculture industry in many parts of the word through the production of vaccines, antibiotics and introduction of bio-security measures to minimize the mass fish mortalities and boots global food fish (Lom and Dykova, 1992).In Ghana, there is currently very little knowledge on the distribution and abundance of pathogens in aquatic ecosystems. This makes it difficult to identify the groups of disease-causing organisms in aquaculture in order to develop preventive and control measures. These concerns about fish diseases and parasitic infestation have existed for years yet little scientific evidence is available on the subject. Parasitic infections of fish can be a major setback in achieving maximum production per unit area of culture. As the development of aquaculture has advanced, one essentially unsolved problem is the prevention of economic losses to production in concrete ponds due to poor management practices which resulted in disease infestation as a result of high prevalence and intensity of parasite load. While this problem remains unsolved, such diseases documented evidence on the prevention and control are apparently deficient.
The study aimed at investigating the presence of fish parasites among Oreochromis niloticus cultured in concrete ponds. Pathogenic effects of parasites on hosts manifest themselves in various ways. According to Huitric et al, (2002) economic effects of parasites on fishes are mass mortality, rejection of infected fish by the market when parasites and/or lesions are visible and more importantly, retarded growth and weight losses of the infected fish. Furthermore, some fish parasites are potentially pathogenic for man when the parasitized fish are consumed not well cooked, cold smoked, marinated or just raw (Naylor et al., 2000).
There are some objectives of the Millennium Development Goals on poverty and hunger. The information related to parasites affecting tilapia fingerlings in concrete ponds would be very important for the implementation of future projects in Tamale, Ghana. Our objective in the present work is to investigate the prevalence and mean intensities of ecto-parasites of Nile tilapia (Oreochromis niloticus).
2 Results
2.1 Physico-chemical Characteristics of pond and Fish Examined for parasites Infestation
The dissolved oxygen (DO) ranged from 4.10 mg/L to 5.12 mg/L with a mean of 4.32 mg/L ± 0.39. The pH ranged from 5.86 to 6.57 with a mean of 6.17± 0.23 and temperature ranged from 27.10℃ to 32.49℃ with a mean of 28.48℃ ± 2.31. Nitrate was 30.00 mg/L to 32.23 mg/L with the mean of 30.63 mg/L ±1.03.
The total number of fish examined was 240 individual fishes for the six month study. Out of the fish sampled for examination, 35% were adult fish while fingerlings were 65%. Out of 240 samples examined, 35 fishes representing (15%) of the total fish examined were parasitized. The number of fingerlings parasitized was slightly higher (0.8%) than the number of adult fishes found to be infected (Table 1). The gills of the fish were observed to have more parasites as indicated in (Table 2). The fish size with the highest (57%) prevalence was 11-13cm. It was followed by fishes with sizes from 14-16 cm, which had a prevalence level of 28%. Low infestations of ecto-parasites were observed at the size ranges of 8 -10 cm and 17-19 cm with prevalence level of 9% and 6% respectively. In terms of prevalence in relation to body weight, the body weight class with the highest prevalence was 37% each for 11-30 g and 31-50 g fish. Body weight class of 51-70 g followed with prevalence of 11% and 9% with the lowest (6%) in weight class of 91-110 g.
|
|
|
|
During the six month study, two protozoan and metazoan ecto-parasites namely Trichodina spp. (Ciliate), monogeneans and Ichthyophthirius multifilis (Ciliate) were found on the gills and the skin mucus of O. niloticus. Out of 240 fish that were examined, 21 were parasitized with Ichthyophthirius multifilis, 8 were found to be infected with Trichodina spp. and 6 were infected with Monogeneans.The peak of infestation was observed to be high in the dry season in February with a decline in the wet season in the month of April (Figure 1).
 Figure 1 The number of parasites recovered from the concrete ponds of Water Research institute Tamale (Nov, 2013- April, 2014) |
2.2 The prevalence of parasites
The prevalence of Trichodina sp. found on gill peaked at 22%. Monogeneans also recovered from the gills peaked at 17% and Ichthyophthirius multifilis recovered from the skin surface peaked at 60% (Figure 2).
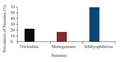 Figure 2 The prevalence of Trichodina, Monogeneans and Ichthyophthirius parasites from concrete ponds at Water Research Institute, Tamale (Nov, 2013- April, 2014) |
2.3 The mean intensity of the parasites
Ichthyophthirius had the highest mean intensity in November and December 2013, and March 2014 whilst Trichodina spp. had the highest mean intensity in January, February and April 2014. The highest mean intensity of 2.5 was observed among the Trichodina spp.in January 2014. The mean intensity of parasites ranged from 0-2.5.The details of the monthly mean intensity of the three parasites are indicated in (Table 3).
|
Table 3 The monthly mean intensity of the three identified parasites Trichodina sp., Monogeneans and Ichthyophthirius |
3 Discussion
The external parasites, viz., ecto-parasites: Trichodina sp, Ichthyophthirius multifilis and Monogeneans were found in relatively low occurrence during the study period as water quality was moderately good except for slightly acidic nature of the pond water. Fish filter ability may be affected by water acidity, evidence in gill susceptibility to ecto-parasite. O. niloticus from the study area exhibited low prevalence and intensities of the external parasites. The prevalence and intensity of parasites recorded indicated that there was less parasite occurrence on the O. niloticus being cultured due to proper management practices. The few occurrence of these ecto-parasites is likely as a result of accidental consequences. The external parasites could be as a result of infected hatcheries during fry production using hapa-in-concrete pond systems. Poor hatchery conditions provide excellent breeding environments for ecto-parasites as intra population transmission rates becomes high in such facilities (Kearn 2004; Wiegertjes and Flik 2004). External parasites from previous stocks which colonized a pond may also be transmitted easily to new stocks which can contribute to an increase in the infestation of parasites when pond water are neither drained when required nor dehydrated out. This may be a common problem for the Institute when there is insufficient water to be used for filling ponds.
In addition, fingerlings have under-developed immune systems, which deliver the natural repellent ability of the gill, fin and skin surface non-functional; and results in increased susceptibility to ecto-parasites which in many cases serve as mechanical vectors to viral and bacterial infections. When such fingerlings are used in stocking any recently established or already existing pond, the parasites may be transmitted into ponds causing an outbreak.
External parasites might be transmitted from infested brood stock from pond to pond through either direct or in direct transition when their fingerlings are used to stock un-infected ponds (Bondad-Reantaso et al., 2005; Murray & Peeler, 2005). There was no clear relationship observed between the intensity of Monogeneans, Trichodina spp. and Ichthyopthirius in the gill and skin mucus samples and temperature in the pond water.
There was no intense variability in both prevalence and intensity of ecto-parasites identified among gills, fin and skin mucus samples from the O. niloticus.
Several works on Trichodina have reported that the organ possesses sucking valves in some species, but hooks are always present. Skin or gill damage from hooks on the opishaptor serves as entry for opportunistic disease causing organisms. According to Klinger (2002), infected fishes lose appetite and scales in areas where flukes are attached. Infected gills may look swollen and pale and have increased respiratory activity (Klinger & Floyd, 2002; Whittington et al., 2000). This might confirm the present outcome in which the Trichodina were more prevalent in the gill than other ecto-parasites.
4 Materials and Methods
Data for this study was collected from Water Research Institute in Tamale, Northern Ghana.The size of concrete ponds in which samples were collected was 5.0 m × 5.3 m and 5.5 m × 5.7 m with water depth of 1m. The study was conducted within six months (November 2013 – April 2014). A total number of 240 Oreochromis niloticus were collected from concrete ponds at Water Research Institute. They were grouped into adult and fingerlings; fingerlings with a total length of 5-11 cm and body weight of 11-12 g and adult with total length of 12 cm – 18cm and weight of 30 g – 100 g. Fish was sent alive in large plastic bags containing pond water to sustain the fish prior to the Laboratory for examination at Water Research Institute Tamale, Ghana.
Samples of fish were taken monthly. The measurement of the total length was done from the tip of the snout to the tip of the caudal fin using a metric measuring board and recorded to the nearest 0.1 cm. The weight of individual fish was taken by singly placing each fish in the pan of the weighing scale. The value for the weight displayed on the screen was recorded to the nearest 0.1 g.
In the laboratory the skin, the skin mucus, the scales, the fins and the gills which form the external parts of the fish were examined for parasites using magnified hand lens. The fishes were examined according to the method described by Paperna (1996). Critical examination was done using light microscope at 40× – 100× for identification. A fish parasitology guide was used for to aid identification of each specimen examined and compared to the plates as described by (Barker et al., 2000).
The data were analysed for the prevalence and mean intensity. The prevalence (%) of the ecto-parasites was estimated as the ratio between the number of infested fish and the number of examined fish expressed in percentages.

The mean intensity (M.I) was determined as the ratio between the total number of parasites in a sample and the number of infested fish in a sample.
![]()
Data obtained from the field and the laboratory results was analysed using statistical tools and descriptions from Microsoft Excel 2013 and Statistica 8.1.
5 Conclusion
Trichodina spp., Monogeneans and Ichthyophthirius multifilis were the ecto-parasites encountered during the study. The prevalence and intensity of the ecto-parasites were low. The effects of slightly acidic pond water on ecto-parasite in fish could be ambiguous, thus requiring concise inferences from further studies. The occurrence of these ecto-parasites had no clear relationship with physico-chemical parameters and it could therefore be as a result of accidental consequences. Both environmental factors and routine management practices carried out on the ponds have no vibrant relationship with the prevalence levels or the intensities of the ecto-parasites.
Acknowledgement
The authors are very grateful to CSIR- Water Research Institute for the provision of the fish sample for the study. The authors wish to express their profound gratitude to the Environmental Chemistry Division of the Institute for the laboratory assistance provided during the study.
Authors’ contributions
Seth Mensah Abobi and Etornyo Agbeko designed the study. Kezia Baidoo performed the statistical analysis, wrote the protocol, and wrote the first draft of the manuscript. Etornyo Agbeko, Seth Mensah Abobi and Kezia Baidoo managed the literature searches and managed the analyses of the study. All authors read, edited and approved the final manuscript.
References
Agbeko E., Kwarfo-Apegyah K., Akongyuure D.N., 2014, Optimization of dugout fisheries for climate Change adaptation in Northern Region, Ghana. Journal of Energy and Natural Resources Management. JENRM, Vol. I, No. 1, 63-68
Al-Rasheid K. A., Ali M. A., Sakran T., Baki A. A. A., and Ghaffar F. A. A., 2000, Trichodinid ectoparasites (Ciliophora: Peritrichida) of some River Nile fish, Egypt. Parasitology International, 49(2):131-137
http://dx.doi.org/10.1016/S1383-5769(00)00042-8
Basson L., and As J., 1994, Trichodinid ectoparasites (Ciliophora: Peritrichida) of wild and cultured freshwater fishes in Taiwan, with notes on their origin. Systematic Parasitology (Historical Archive) 28: 197-222
http://dx.doi.org/10.1007/BF00009518
Barker D. E., and Cone D. K., 2000, Occurrence of Ergasilus celestis (Copepoda) and Pseudodactylogryrus anguillae (Monogenea) among wild eels (Anguilla rostrata) in relation to stream flow, pH and temperature and recommendations for controlling their transmission among captive eels. Aquaculture 187: 261-274
http://dx.doi.org/10.1016/S0044-8486(00)00324-0
Bondad-Reantaso M. G., Subasinghe R. P., Arthur J. R., Ogawa K., Tan Z., and Shariff M., 2005, Disease and health management in Asian aquaculture. Veterinary Parasitology 132: 249-272
http://dx.doi.org/10.1016/j.vetpar.2005.07.005
FAO, 2005, Aquaculture production, (2004, Year book of Fishery Statistics - Vol.96/2. Food and Agriculture organization of the United Nations, Rome, Italy.
Huitric M., Folke C., and Kautsky N., 2002, Development and government policies of the shrimp farming industry in Thailand in relation to mangrove ecosystems. Ecological Economics 40: 441-455
http://dx.doi.org/10.1016/S0921-8009(02)00011-3
Jadwiga, 1991, Producers for water and waste water analysis, Loveland (CO): Jadwiga Chemical Co.
Kearn G.C., 2004, Leeches, Lice and Lampreys: A Natural History of Skin and Gill Parasites for Fishes. Springer, Dordrecht, the Netherlands.
Klinger R., and Floyd R. F., 2002, Introduction to Freshwater Fish Parasites 1.
Lom J., and DYkova I., 1992, Protozoan parasites of fishes. Elsevier Science Publishers.
Lom J., 1995, Trichodiniae and other Ciliates (Phylum Ciliophora) Pages 229-262 in P. T K. Woo, editor. Fish Diseases and Disorders: Protonoan and metazoan infections. CAB International, Cambridge.
Murray A.G., and Peeler E.J., 2005, A framework for understanding the potential for emerging diseases in aquaculture. Preventive Veterinary Medicine 67: 223-235
http://dx.doi.org/10.1016/j.prevetmed.2004.10.012
Naylor A. G., and Peeler E. J., 2005, A framework for understanding the potential for emerging diseases in aquaculture. Preventive Veterinary Medicine 67: 223-235
http://dx.doi.org/10.1016/j.prevetmed.2004.10.012
Pozio E., and Rosa G. L., 2005, Evaluation of the infectivity of Trichinella papuae and Trichinella zimbabwensis for equatorial freshwater fishes. Veterinary Parasitology 132: 113-114
http://dx.doi.org/10.1016/j.vetpar.2005.05.038
Paperna I., 1996, Parasites, infections and diseases of fishes in Africa: An update. Page 232 in F. D. Repository, editor. CIFA TEchnical Papers- CIFA/T31 Hebrew Univ. of Jerusalem, Rehovot (Israel).
Van As J. G., and Basson L., 1987, Host specificity of trichodinid ectoparasites of freshwater fish. Parasitology Today 3: 88-90
http://dx.doi.org/10.1016/0169-4758(87)90166-9
Whittington I. D., and Cribb B. W., Hamwood T. E., and Halliday J. A., 2000, Hostspecificity of monogenean (platyhelminth) parasites: a role for anterior adhesive areas? International Journal for Parasitology 30: 305-320
http://dx.doi.org/10.1016/S0020-7519(00)00006-0
Wiegertjes G. F., and Flik G., 2004, Host-Parasite Interactions. Garland Science/BIOS Scientific Publishers, The Netherlands
. PDF(464KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. K. Baidoo
. S.M. AbobiBaidoo
. E. Agbeko
Related articles
. Oreochromis niloticus
. Intensity
. Prevalence
. Tamale
Tools
. Email to a friend
. Post a comment


