The Barbels of the Adult African Catfish from Eastern Nigeria: a Micro Morphological and Functional Study 
 Author
Author  Correspondence author
Correspondence author
International Journal of Aquaculture, 2015, Vol. 5, No. 21 doi: 10.5376/ija.2015.05.0021
Received: 30 Apr., 2015 Accepted: 23 Jul., 2015 Published: 27 Jul., 2015
Ikpegbu E. and Nlebedum U.C., 2015, The Barbels of the Adult African Catfish from Eastern Nigeria: a Micro Morphological and Functional Study, International Journal of Aquaculture, 5(21): 1-6
The micro-morphology of adult farmed African catfish Clarias gariepinus barbel was investigated to enrich our knowledge of teleost biology as there is dearth information on this species barbel from available literature, and also for any functional morphological adaptation. After humane immobilization of the animal, the sample under study- barbels were dissected out and processed for routine histology. The histology revealed variation in the components of its root, stem and tip especially the relative size of the elastic cartilage core and surrounding connective tissue coat. The epithelium was of the stratified squamous cells containing mucous cells, taste buds, melanocytes and club cells. Beneath the epithelium was varying layers of highly vascularized and innervated connective tissue surrounding the linearly arranged elastic cartilage core. This micro-architecture can be related an organ involve in both mechanoreceptor through extensive innervations and chemoreceptor by means of the taste bud gustatory apparatus. The elastic cartilage core will enhance flexibility during movement while the club cells will help in flight or fight. The histology from this study suggest that the barbel will aid guide the fish in movement especially avoiding obstacles and predators, help in food search and selection through gustation.
Introduction
Morphologically, the structure barbel is used to describe tentacular sensory podia-like organs in some vertebrates like the fishes, amphibians and reptiles (Winokur, 1982; LeClair and Topczewski, 2010; In-Seok et al., 2012). In most teleosts, barbels are skin appendages used for gustation and mechanoreception (Griswold, 1972). The number and shape of Barbel are highly inconsistent, with some species having up to 10 paired or unpaired barbels on various areas of the jaws, lips and head (Eakin et al., 2001; Park et al., 2005). Intra species barbel sexually dimorphism or polymorphism amongst fish of either sex has been reported (Eakin et al., 2006). From reported morphological descriptions, the teleost barbel contains an outer epithelium, dermal connective tissue, blood vessels, and extensions of the facial nerves that innervate numerous taste buds (Dimmick, 1988; McCormick and Shand, 1992; Sakata et al., 2001; Kiyohara et al., 2002). Variable features include a central rod of connective tissue or cartilage, and intrinsic and/or extrinsic muscle groups that allow the barbel some range of motion. Although once used as a taxonomic character to unite all “fish with whiskers”, barbels are now believed to be phylogenetically undependable having been gained or lost frequently in many genera (Broilay et al., 1998; Arai and Kato, 2003).
The morphology of the barbell has been documented in some fish like striped sea catfish Plotosus lineatus (Park et al., 2005); an Hawaiian goatfish (Holland, 1976); Callichrous bimaculatus, Heteropneustes fossilis, Clarias batrachus and Rita rita (Satō and Kapoor, 1957; Srivastava and Sinha, 1961; Singh and Kapoor, 1967); Channel catfish Ictalurus punctatus (Joyce and Chapman, 1978); the ability of the barbel to regenerate is also in literature like the Parasilurus asotus, silurid fish Corydora aeneus, and Chinese Longsnout Catfish Leiocassis longirostris, (Sato and Katagiri, 1966; Shiba et al., 1982; In-Seok et al., 2012), but there is dearth of information from available literature on the barbel histology from the farmed African catfish. Hence, the aim of this work is to study its micro-morphology, fill the knowledge gap, and possibly correlate morphology to organ function especially adaptive features.
Materials and Methods
Seven adult African catfish sourced from a commercial fish farm- Yuep in Umuahia area of Eastern Nigeria were used for the study. Their mean weight and length were 30.76 ± 3.30 g and12.03 ± 0.27 cm respectively. The fish were humanely immobilized with mild chloroform sedation and stunning. The specimen under study – the barbel was excised from the upper lip of the cranial region. Sections of the root, stem or middle piece and tip or extremity were immediately fixed in 10% neutral buffered formalin.
The tissues were passed through graded ethanol, cleared in xylene, impregnated and embedded in paraffin wax. Sections 5µm thick were obtained with Leitz microtome model 1512. They were stained with haematoxylin and eosin for light microscopy examination (Bancroft and Stevens, 1977). Glyco-conjugates were demonstrated using a periodic acid Schiff (PAS) procedure with and without prior digestion with diastase (Lillie and Greco, 1947; Ikpegbu et al., 2011). Photomicrographs were taken with – Moticam 2001 camera (Moticam UK) attached to Olympus microscope.
Results
Grossly the dark coloured barbel was divided into three parts for better appreciation of its physiology and anatomy. The region inserted in the lip was referred to as the root, stumpier section following the root was called the stem or middle piece, while the extreme was referred to as the tip.
At the root of the barbel in the cavity of the lips, the base was seen enlarged and bulging, giving the barbel a club-shaped appearance (Figure 1). Thus a longitudinal section of the barbell revealed a bulging base or root, slender middle piece or stem and a tapering tip or extremity. The root contained an elastic cartilage core surrounded by a slightly enlarged rim of smooth muscle fibres. This smooth muscle wall was in close contact with skeletal muscle bundles, loose connective tissue, nerve fibre and small blood vessels.
At the middle piece or stem of the barbell, the histology of the longitudinal section revealed a covering epidermis with an epithelium of stratified squamous cells containing PAS positive mucous cells, taste buds, melanophores and eosinophilic club cells (Figure 2). The dermal region contained mainly dense irregular connective tissue, blood vessels, melanocytes, melanophores, nerve fibres and very small areas of isolated loose connective tissue. This dermal layer made few lateral invasions into the epithelium, and such extensions appeared like buds or papillae covered by single layer of squamous cells (Figure 3). The layer beneath the dermis contained smooth muscle fibres and it directly covered the elastic cartilage core. The elastic cartilage core was a linear structure that occupied the centre of the barbel middle piece. A transverse section of the barbel stem at lower magnification revealed that the oval-shaped circumference was not smooth due to the extensions already described. The two subepithelial regions already described in the longitudinal section were also observed. In addition to the elastic cartilage core, a nerve fasciculus was seen (Figure 4). This nerve trunk was lined by simple squamous cells. The wall of the nerve trunk was composed of dense regular connective tissue containing blood vessels. Some blood vessels bulged into nerve trunk (Figure 4).
The micro-architecture of the barbel tip was somewhat reverse of that observed at the stem. The epidermis was thinner than that of the stem. The dermis was half the thickness of the smooth muscle, while the cartilage core was larger than the size of the dermis and smooth muscle layer put together (Figure 5, 6). The tip of the barbel was the most vascularized section of the organ but least innervated (Figure 6).
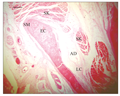 Figure 1 Longitudinal section of the barbel root showing the elastic cartilage core EC, surrounded by smooth muscle fibres SM. Note that club shaped barbel root is in close contact with skeletal muscle SK, adipose tissue AD, and loose connective tissue LC. H&E. (Scale bar = 50 µm) |
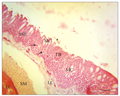 Figure 2 Transverse section of the barbel showing epidermal epithelium SSE, containing mucous cells MC, taste bud TB, and eosinophilic club cells (black arrow). Note the dermal loose connective tissue LC, smooth muscle coat SM, of the elastic cartilage EC. H&E. (Scale bar = 25 µm) |
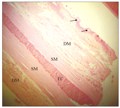 Figure 3 Longitudinal section of the barbel stem showing dermal loose connective DM, smooth muscle fibres SM, elastic cartilage core EC. Note the dermal loose connective tissue out-pocketing into the epidermis (white arrow). Observe the relative sizes of DM, SM and EC. H&E. (Scale bar = 100 µm) |
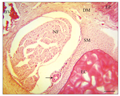 Figure 4 Transverse section of the barbel stem showing the epidermis EP, dermis DM, blood vessels BV, large nerve fibre NF, elastic cartilage EC, smooth muscle fibres SM. Note epimysium blood vessel (white arrow). H&E. (Scale bar = 25 µm) |
.png) Figure 5 Longitudinal section of the barbel tip showing thin epidermal/dermal layer (white arrow), smooth muscle fibres SM, elastic cartilage core EC. Observe the relative sizes of the layers (Scale bar = 100 µm) |
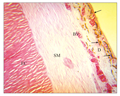 Figure 6 Longitudinal section of the barbel tip showing epidermis (black arrow); dermis D, containing melanphores (white arrow), abundant blood vessels BV. Note the smooth muscle coat SM, elastic cartilage core EC. Observe the relative sizes of D, SM and EC. H&E. (Scale bar = 50 µm) |
Discussion
The histology of the African catfish barbel from this confirms that the barbel is an appendage of the skin by possessing similar basic features of the skin like well developed epidermis and dermis. The presence of stratified squamous epithelium in the epidermis will help protect the underlying structures. This type of epithelium has been reported in the Channel catfish, Ictalurus punctatus (Joyce and Chapman, 1978). In the Zebrafish, the epidermis of the maxillary barbel is of stratified cuboidal epithelium (LeClair and Topczewski, 2010). The mucous cells will produce mucin that protects the organ from mechanical abrasion (Singh and Kapoor 1967; Khojasteh et al., 2009). When acid mucins are elaborated, it protects the organ against pathogens like bacteria present in the environment (Elbal and Agulleiro, 1986; Neuhaus, et al., 2007)). Mucous cells have been reported in the barbels of Zebrafish (LeClair and Topczewski, 2010). The taste buds on the epidermis will locate food in distant places, thus help in guiding foraging activities for food (Moyle and Cech, 2000). This quality has made fish biologist refer to it as an accessory feeding structure (In-Seok et al., 2012), but the actual selection by gustation before ingestion is done in the oropharyngeal cavity by means of the pharyngeal pad (Ikpegbu et al., 2012). The presence of taste buds have been reported in the Leiocassis longirostris where it is also associated with enhanced chemoreception (In-Seok et al., 2012); and in the Blepsias cirrohosus draciscus (Satō, 1977).
The eosinophilic club cells also referred to as alarm cells are for flight or fight response in the presence of predator (Chivers and Smith, 1998; Brown, 2003). For most period of the last century, it was believed that, the club cells are basically limited to the alarm function. But, the presence of the eosinophilic club cells of fishes has been of major interest to evolutionary ecologists because the discharge of the mediators alarm response are under involuntary control, and does not takes place unless the fish is wounded (Ahmed and Imam, 2011). Current reports are associating the club cells with immune response to invading pathogens, penetrating parasites, epidermal injury and exposure to environmental stress factors like ultraviolet radiation (Chivers et al., (2007; Halbgewachs et al., 2009). Their recent investigation is suggesting that the emergence of alarm function by club cells is secondary to their primary function of being an integral component of fish inherent immune system. The presence of these barbel club cells have been documented in these fish Callichrous bimaculatus, Heteropneustes fossilis, Clarias batrachus and Rita rita (Satō and Kapoor, 1957; Srivastava and Sinha, 1961; Singh and Kapoor, 1967).
The presence of abundant vascularization indicates an active organ that needs adequate supply of oxygen and metabolites for optimum functioning. The nerve bundle is involved in mechanoreception and the presence of other several smaller nerve ramifications suggests a very tactile organ that is very sensitive touch. The essential role of barbel nerves in the regerneration of the taste buds is in literature (Kamrin and Singer, 1955). The abundant loose connective tissue will help in easy movement of connective tissue cells and aid the response of the barbell to challenges in the environment. The melanophores in the epidermis and dermis are pigments responsible for the dark gross colouration of the barbel. Their abundance may reflect need to shield underlying structures from the effects of ultraviolet rays in the tropical environment. It is believed that extensive presence of melanocytes and melanophores in some organs may infer some phagoc- ytic activity that helps in body defense (Le Poole et al., 1993). The presence of melanocytes in other fish barbel is in literature (LeClair and Topczewski, 2010).
The elastic cartilage core serves as the supporting rod of the barbel. The presence of this cartilage and taste buds makes this barbel under study a stiff but flexible gustatory type associated with location of prey in catfishes (Kapoor and Bhargava, 1967; McCormick and Shand, 1992; Park and Kim, 2005). The cartilage rod has been reported in the Channel catfish, Ictalurus punctatus (Joyce and Chapman, 1978). The presence of smooth muscle surrounding the elastic cartilage suggests that the movement of the barbel is under involuntary control, helping the organ to quickly direct the fish away from possible source of mechanical trauma. The involuntary control also helps explain the presence of elaborate nerve fibre ramification. The barbel is believed to be innervated by facial cranial nerve.
The variation in the relative size of the epidermis, dermis, smooth muscle coat and elastic cartilage core as observed in this stem and tip, may suggest the need for better connective tissue coat at the stem while the tip requires bigger cartilage and smooth muscle cells for ease of flrxible movement in murky swamps or penetration in mud, as the fish also inhabits in mud during unfavourable environmental conditions like low water content in swamps to avoid desiccation.
In conclusion, this study that fills the existing knowledge gap of barbel histology of the African catfish suggests an organ well adapted for chemoreception and mechanoreception. The barbel by means of taste buds present effectively guides the fish towards source of food even in the complete absence olfactory response by the olfactory rosette (Holland, 1976). The club cells warn of danger in the vicinity and also help in body defense. This organ from this study is an important skin appendage that must have evolved to help the fish survive in swampy or muddy environment. The knowledge obtained will enhance our understanding of the African catfish biology and morpho-functional adaptive mechanism.
References
Arai R., and Kato K, 2003, Gross morphology and evolution of the lateral line system and infraorbital bones in bitterlings (Cyprinidae, Acheilognathinae), with an overview of the lateral line system in the family Cyprinidae. Bulletin of the University Museum, University of Tokyo, 40: 1-42
Ahmed S.A.H., and Imam A.A., 2011, Skin Characteristics and Organization of the Air-breathing Fish, Alticus kirkii (Günther, 1868) along Different Body Regions. Journal of Biological Sciences, 11: 466-474
http://dx.doi.org/10.3923/jbs.2011.466.474
Briolay, J; Galtier, N; Brito, R. and Bouvet, Y., 1998, Molecular phylogeny of Cyprinidae inferred from cytochrome b DNA sequences. Molecular Phylogenetic Evolution, 9: 100-108
http://dx.doi.org/10.1006/mpev.1997.0441
Bancroft, J.D. and Stevens, A., 1977, Theory and Practice of Histological Techniques. Churchill Livingstone, New York, USA, p88-89
Brown, G.E., 2003, Learning about danger: Chemical alarm cues and local risk assessment in prey fishes. Fish Fishries, 4: 227-234
http://dx.doi.org/10.1046/j.1467-2979.2003.00132.x
Chivers, D.P. and Smith, R.J.F., 1998, Chemical alarm signaling in aquatic predator-prey systems: A review and prospectus. Ecoscience, 5: 338-352
Chivers, D.P; Wisenden, B.D; Hindman, C.J; Michalak, T.A. and Kusch R.C; et al., 2007, Epidermal alarm substance cells of fishes are maintained by non-alarm functions: possible defence against pathogens, parasites and UVB radiation. Proceedings of Royal Society of Biological Sciences Series B, 274: 2611-2619
http://dx.doi.org/10.1098/rspb.2007.0709
Dimmick, W.W., 1988, Ultrastructure of North American cyprinid maxillary barbels. Copeia 72-80
http://dx.doi.org/10.2307/1445924
Eakin, R.R; Eastman, J.T. and Jones, C.D., 2001, Mental barbel variation in Pogonophryne scotti Regan (Pisces: Perciformes: Arted idraconidae). Antarctic Science, 13 (4): 363-370
http://dx.doi.org/10.1017/S0954102001000517
Eakin, R.R; Eastman, J.T and Vacchi, M., 2006, Sexual dimorphism and mental barbel structure in the South Georgia plunderfish Artedidraco mirus (Perciformes : Notothenioidei : Artedidraconidae). Polar Biology, 30: 45-52
http://dx.doi.org/10.1007/s00300-006-0158-x
Elbal, M.T. and Agulleiro, B.A., 1986, Histochemical and Ultrastrucutral study of the gut Mugil saliens (teleost). Acta Microscopic, 9 (1): 31-40
Griswold, R.E., 1972, "Investigation of barbel regeneration in the catfish Ameiurus nebulosus". Senior Scholar Papers. Paper 108.
http://digitalcommons.colby.edu/seniorscholars/108. Downloaded December 28, 2013, 3:00pm.
Halbgewachs, C.F; Marchant, T.A; Kusch R.C. and Chivers, D.P., 2009, Epidermal club cells and the innate immune system of minnows. Biological Journal of Linnean Society ,98: 891-897
http://dx.doi.org/10.1111/j.1095-8312.2009.01328.x
Holland, K. N., 1976, A behavioral and electrophysiological investigation of the taste function of the barbels of an Hawaiian goatfish. MSc Thesis, University of Hawaii, p 59
Ikpegbu, E; Nlebedum, U.C; Nnadozie, O. and Agbakwuru, I.., 2011, Fast Green FCF or Ehrlich’s hematoxylin as counterstain to periodic acid Schiff reaction: A comparative study. Histologic, 54: 29-30
Ikpegbu, E; Ezearsor, D.N; Nlebedum, U.C; Nwogu, C; Nnadozie, O. and Agbakwuru I.., 2012, Histological study of the pharyngeal pad of the African catfish (Clarias gariepinus Burchell, 1822). Animal Research International, 9(3): 1613-1618
In-Seok, P; Chi-Hong, K. and Choi, J.W., 2012, Histological Observations and Regeneration of Barbels in Juveniles of the Chinese Longsnout Catfish Leiocassis longirostris. Fisheries Aquatic Sciences, 15(4): 299-303
http://dx.doi.org/10.5657/FAS.2012.0299
Joyce, E.C. and Chapman, G.B., 1978, Fine structure of the nasal barbel of the channel catfish, Ictalurus punctatus. Journal of Morphology, 158(2):109-53
http://dx.doi.org/10.1002/jmor.1051580202
Kamrin, R.P. and Singer, M., 1955, The influence of the nerve on regeneration and maintenance of the barbel of the catfish, Ameiurus nebulosus. Journal of Morphology, 96: 173-187
http://dx.doi.org/10.1002/jmor.1050960108
Khojasteh, S.M.B; Sheikhzadel, F; Mohammadnejad, D. and Azani, A., 2009, Histological, Histochemical and ultrastructural study of the intestine of Rainbow trout (Oncorhynchus mykiss). World Applied Science Journal, 6: 1525-1531
Kiyohara, S; Sakata, Y; Yoshitomi, T. and Tsukahara, J., 2002, The ‘goatee’ of goatfish: Innervation of taste buds in the barbels and their representation in the brain. Proceedings of Royal Society of Biological Sciences Series B, 269: 1773-1780
http://dx.doi.org/10.1098/rspb.2002.2086
Kapoor, B.G. and Bhargava, S.C., 1967, A study on the barbels of a marine catfish, Arius thalassinus (Rüpp.). Japan Journal of Ichthyology, 14: 201-206
Le Poole, I.C; Van den Wijngaard, R.M; Westerhof, W; Verkruisen, R.P; Dutrieux, R.P; Dingemans, K.P. and Das, P.K., 1993, Phagocytosis by normal human melanocytes in vitro. Experimental Cellular Research, 205(2): 388-395
http://dx.doi.org/10.1006/excr.1993.1102
Lillie, R.D. and Greco, J., 1947, Mact diastase ptyalin in place of saliva in the identification of glycogen. Staining Technique, 22: 67-70
LeClair, E.E. and Topczewski, J., 2010, Development and Regeneration of the Zebrafish Maxillary Barbel: A Novel Study System for Vertebrate Tissue Growth and Repair. PLoS ONE, 5(1): e8737
http://dx.doi.org/10.1371/journal.pone.0008737
McCormick, M.I. and Shand, J., 1992, Metamorphosis of the Visual and Barbel Sensory Systems at Settlement in the Reef Fish Upeneus tragula (Family Mullidae). Proceedings of the Seventh International Coral Reef Symposium, Guam, 1: 616-624
Moyle, P.B. and Cech, J.J. Jr., 2000, Fishes: An Introduction to Ichthyology. Prentice-Hall Inc., Upper Saddle River, NJ, US
Neuhaus, H; Marel, M; Caspari, N; Meyer, W; Enss, M.L. and Steinhayen, D., 2007, Biochemical and histochemical study on the intestinal mucoda of the common carmp Cyprinus carpio L. with special consideration of mucin glycoproteins. Journal of Fish Biology, 70: 1523-1534
http://dx.doi.org/10.1111/j.1095-8649.2007.01438.x
Park, I.S; Seol, D.W; Kim, E.M; Kim, Y.J. and Lee, Y.D., 2005, Histology of the barbels of striped sea catfish Plotosus lineatus (Thunberg). Journal of Korean Fish Society, 38: 158-163
http://dx.doi.org/10.5657/kfas.2005.38.3.158
Park I.S. and Kim C.H.., 2005, Charactersistics of the histological structure of the mandibular barbels of two species of catfish (Siluridae) from Korea. Korean Journal of Ichthyology, 17, 36-42
Sakata, Y; Tsukahara, J. and Kiyohara, S., 2001, Distribution of nerve fibers in the barbels of sea catfish Plotosus lineatus. Fisheries Science (Tokyo), 67: 1136-1144
http://dx.doi.org/10.1046/j.1444-2906.2001.00371.x
Satō, M., 1977, Histology of barbels of Blepsias cirrhosus draciscus (Cottidae). Japan Journal of Ichthyology, 23: 220-224
Sato, M. and Kapoor, B. G.(1957, “Morphological and Histological observations on the barbels of a few Indian freshwater fishes, Alaska Codfish and Podothecus cipenserinus,” Anatomy and Zoology Journal of Japan, 30, 156-61
Sato, M. and Katagiri, Y., 1966, Regeneration of the mandibular barbels of the fry of the catfish, Parasilurus asotus, and comparison of histological structure of the mandibular barbels of three catfishes inhabiting Lake Biwa. Japan Journal of Ichthyology, 13: 169-175
Shiba, Y; Sasaki, Y; Enomoto, T. and Kanno, Y., 1982, Microtubule formation in regenerating terminal buds of the silurid fish, Corydora aeneus. Develop. Growth and Differenciation, 24: 199-203
http://dx.doi.org/10.1111/j.1440-169X.1982.00199.x
Singh, C.P. and Kapoor, B.G., 1967, Histological observations on the barbels of a bagrid catfish Rita rita (Ham.). Japan Journal of Ichthyology, 14: 197-200
Srivastava, C.M. and Sinha, A.N., 1961, Contribution to the study of barbels of fishes. Acta Society Zoology Bohem, 25: 12-15
Winokur, R.M., 1982, Integumentary appendages of Chelonians. Journal of Morphology, 172: 59-74
http://dx.doi.org/10.1002/jmor.1051720106
. PDF(524KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Ikpegbu E.
. Nlebedum U.C
Related articles
. Barbel
. Mechanoreceptor
. Chemoreceptor
. Histology
. African catfish
Tools
. Email to a friend
. Post a comment


