Probiotic Potential of Gut Associated Bacteria from Indigenous Fresh Water Ornamental Fishes of Kerala, South India 
2 Nansen Environmental Research Centre, India (NERCI), 6A, Oxford Business Centre (6th Floor), Sreekandath Road, Ravipuram, Kochi 682016, Kerala, India
 Author
Author  Correspondence author
Correspondence author
International Journal of Aquaculture, 2015, Vol. 5, No. 16 doi: 10.5376/ija.2015.05.0016
Received: 15 Apr., 2015 Accepted: 23 Jul., 2015 Published: 01 Jul., 2015
Nashad M., Mujeeb Rahiman K.M., Ajin A.M and Mohamed Hatha A.A., 2015, Probiotic Potential of Gut Associated Bacteria from Indigenous Fresh Water Ornamental Fishes of Kerala, South India, International Journal of Aquaculture, 5(16): 1-6 (doi: 10.5376/ija.2015.05.0016)
Probiotic potential of gut associated aerobic and facultative anaerobic bacterial flora of indigenous freshwater fishes such as Puntius filamentosus and Barilius bakeri, were analysed in this study. Total viable count (TVC) of heterotrophic bacteria ranged between 0.64 x 107 to 1.31 x 107 and 0.59 x 107 to 1.92 x 107 per gram in gut of Barilius bakeri and P. filamentosus respectively. While bacteria belonging to the genus Corynebacterium dominated the gut of P. filamentosus, Bacillus was found to be dominant genus in the gut of B. bakeri. More than 50% of bacterial isolates from both these fishes were capable of producing various exoenzymes such as amylase, gelatinase and lipase, with 15% of them showing excellent amylolytic and gelatinolytic activity. Selected bacterial isolates were tested for antagonistic activity against fish, shrimp and human pathogens, which revealed 15% of isolates having antagonistic activity against at least one pathogenic Vibrio species tested. These isolates were further tested for their ability to grow under different temperature, pH and salinity conditions in order to evaluate their suitability for application under different filed conditions. The result of the present study offer scope for further research to evaluate probiotic potential of these gut associated bacteria in the larval rearing system and hatchery operations.
Introduction
Ornamental fish keeping is one of the most popular hobbies of the world today. With the increase in demand for ornamental fishes especially in developed countries, many countries in Asia have started farming and nearly 60% of the international trade in ornamental fishes originates from developing countries. The export of ornamental fishes from India is at present mainly confined to freshwater varieties and the export is limited to fishes collected from wild (Madhu et al., 2009). More than 230 fish species have been reported from various river systems of Kerala and almost half of them are ornamental species (Radhakrishnan and Kurup, 2006). Extensive collection of these fishes from wild stock resulted in the decline of fish diversity in riverine system.
Hatchery rearing is an alternative to overcome this situation. However, disease outbreaks during the early developmental stages results in economic loss and is the main constraint in preventing the success (Verschuere et al., 2000). Controlling diseases through antibiotic treatment is also leading to the emergence of drug resistant pathogens, which are difficult to treat in the long run. In recent years, control of diseases by environment friendly methods such as probiotic bacteria and immunostimulants has gained attention of researchers (Castex et al., 2008). The use of probiotic bacteria to control potential pathogens is showing good results (Gomez- Gil et al., 2000).
Kerala is blessed with 42 rivers and most of them support unique fish fauna, which include potential ornamental fishes. Considering this potential, government of Kerala has also initiated steps to promote ornamental fish culture in small scale house hold ponds and tanks. As a precondition to commercial exploitation, techniques need to be developed for successful breeding and rearing of potential indigenous ornamental fishes. In the present study fishes such as Puntius filamentosus and Barilius bakeri, which have ornamental value, were chosen to study their gut microbiota and to evaluate their probiotic potential. The study has been taken on the assumption that an understanding of the gut microbiota of these indigenous ornamental fishes, might lead to valuable insight for the development of possible strains that could be used as probiotics in the breeding, larval rearing, and growth enhancement.
1 Materials and Methods
1.1 Description of the Collection site and Fishes selected for the Study
Fresh water fishes namely Puntius filamentosus (Filament Barb) and Barilius bakeri (Malabar Baril) (Plate 1b & c) were collected from its natural habitat Chalakudi River, Latitude: 10 09’ 44’ N; Longitude: 76 15’ 56’’E (Plate 1 a) and brought alive to the laboratory. Fishes were collected with the help of professional fisherman, who caught them using cast net. After taking the morphometric measurements such as total length (TL) and standard length (ST) the fishes were dissected out aseptically using sterile surgical blade. The entire gut region was aseptically removed, weighed and homogenized using sterile glass homogenizer, and serially diluted up to 10-6 using 10% phosphate buffer solution of pH 7.2.
Aliquots of 0.2 ml samples from each dilution were spread plated in duplicate on nutrient agar with media composition of peptic digest of animal tissue and NaCl - 5g/L each, Beef extract and Yeast extract - 1.5 g/L each, Agar – 15g/L (Himedia- Mumbai) for the enumeration of cultivable bacterial flora aerobic heterotrophic bacteria. The plates were then incubated at 30℃ for 24 hours. Colonies developed on the plate were counted and expressed as colony forming units (cfu) /mL of fish gut. Well separated and morphologically different colonies were picked up using a sterile inoculation needle and transferred to sterile nutrient agar slants. The isolates were purified by quadrant streaking and were stored in nutrient agar slants for the further study. Estimation of microbiota of the gut was done using standard methods (Ringø et al., 1995). The isolated bacterial strains were identified up to generic level using the taxonomic key by Buchanan & Gibbons (1984). For generic level classification of the isolates they were subjected to various tests such as Gram stain, spore stain, motility, Kovacs oxidase test, catalase activity, and oxidation / fermentation test along with morphological characteristics of the colony.
1.2 Hydrolytic Enzyme production potential of the isolates
Ability of the gut associated bacteria from P. filamentosus and B. bakeri to produce various hydrolytic enzymes such as amylase; lipase and gelatinase were checked by plate assay. For amylase and gelatinase activity, nutrient agar plates with 1% soluble starch and 1% gelatin respectively were prepared and the cultures were spot inoculated in the plates. After incubation of the plates at room temperature (28 + 2℃) for 48 h, nutrient agar plates with 1% soluble starch were flooded with Lugol’s iodine and nutrient agar plates with 1% gelatin flooded with mercuric chloride solution (Frazier, 1926). The presence of a clear zone around the colonies was noted as a positive result in both tests. For lipase activity, nutrient agar plates with 1% Tween-80 were used. Test organisms were spot inoculated and plates were incubated at room temperature for 3-4 days. A positive result was indicated by zone of clearing around the colonies of lipolytic organisms (Rhodes, 1959).
1.3 Determination of Antibacterial activity of the Isolates
Antibacterial activity of heterotrophic bacteria isolated from the gut of P. filamentosus and B. bakeri were determined following the method described by (Mujeeb et al., 2010). The antibacterial activity was assayed by disc diffusion method against different fish, shrimp and human pathogens such as Vibrio harveyi, V. alginolyticus, V. splendidus, V. flluvialis, V. cholera, Pseudomomas aeruginosa, Aeromomas hydrophila, A. caviae and Salmonella strain 14 and 44. Filter paper discs of 6 mm diameter were cut out from Whatmans No. 1 filter paper and sterilized at 15 lbs for 15 min. The bacterial isolates were enriched in nutrient broth by overnight incubation at 37℃ for 16 hours. The filter paper discs were then impregnated with 20 µl of broth cultures of the test organisms. These discs were then placed over sterile tryptic soy agar (TSA) seeded with test pathogen. The seeded plates along with test culture were then incubated at 37℃ for 24 hours. Formation of clearing zone around the discs was considered positive indication of inhibitory activity.
1.4 Determination of Growth abilities of the Selected Isolates
Growth of any organism depends on the certain physical and chemical parameters, and each organism has a minimum and maximum range of tolerance beyond which, their growth is inhibited. Metabolic activity of bacteria is directly linked to the environmental parameters like temperature, salinity, and pH (Monod, 1949). Isolates showing good enzyme activity were selected for the growth studies. Isolate numbers T3, T6, T8, T14 and T23 were selected from B. bakeri and P3, P7, P8, P14 and P20 from P. filamentosus.
In order to check the effect of temperature on growth, these isolates were then inoculated into 5ml sterile nutrient broth (pH 7) and incubated at various temperatures such as 4, 20, 27, 37 and 50℃. Similarly effect of pH on growth of these isolates was monitored by inoculating the cultures into sterile nutrient broth prepared with various pH such as 2, 4, 6 and 8. Ability to grow in various salinity was tested similarly by monitoring the growth in nutrient broth maintained at various salinities such as 0, 10, 15 and 25 ppt. The inoculated nutrient broth tubes for checking the effect of pH and salinity were incubated at (28 + 2℃). Growth is measured in terms of optical density at 620 nm, after 10, 24 and 48 hours.
2 Results and Discussion
2.1 Enumeration and Characterisation of Gut bacteria
Total viable count (TVC) of heterotrophic bacteria ranged between 0.64 x 107-1.31 x 107 cfu/mL in the gut of Barilius bakeri, 0.59 x 107 -1.92 x 107 cfu/mL guts of the fish P. filamentosus. A total of fifty eight isolates were selected for characterisation and further analysis from the fish gut samples, (32 from the B. bakeri and 26 from the P. filamentosus). Characterisation of the isolates revealed that Corynebacterium is the predominant genus in the gut of P. filamentosus followed by genera such as Kurthia, Micrococcus and Staphylococcus. Bacillus sp. was found to be the dominant genera in the gut of B. bakeri followed by genera Corynebacterium, Micrococcus, Planococcus, Kurthia and Staphylococcus. (Figure 1). In both these fishes the gut bacterial flora was found to be dominated by Gram positive forms. These results are in agreement with the microbiota that has been reported previously from the guts of different species of fishes (Ringø et al., 1998; Ray et al., 2012). Both marine and freshwater water fish have been shown to have a specific indigenous gut microbiota and it may change with age, nutritional status, and environmental conditions (Olafsen, 2001; Vine et al., 2006). In general, indigenous microbiota of freshwater fish species tends to be dominated by members of the genera Aeromonas,Plesiomonas, representatives of the family Enterobacteriaceae, and obligate anaerobic bacteria of the genera Bacteroides, Fusobacterium, and Eubacterium (Sakata, 1990). However, the aerobic heterotrophic bacteria of the gut of P. filamentosus and B. bakeri were found to be dominated by Gram positive forms. These variations are accounted due to factors like bacterial host specificity, food type, and water resource (Verner et al., 2003).
.png) Figure 1 Distribution of various genera of heterotrophic bacteria in the gut of a) P. filamentosus and b) B. bakeri |
2.2 Hydrolytic enzyme production potential of the Bacterial isolates from Fish Gut
These diverse bacterial flora of the gastrointestinal tract represent a diversified enzymatic potential. Resident intestinal bacteria in fish are known to accelerate the digestion process by producing extracellular enzymes (Stickney and Shumway 1974; Cahill, 1990; Subha, 2013). Understanding of the enzyme producing potential of gut microbiota may help in formulating feeds and probiotics for larval rearing. All isolates were screened for their ability to produce hydrolytic enzymes such as amylase, gelatinase, and lipase. Results revealed widespread hydrolytic enzyme production potential among the isolates from B. bakeri (Figure 2). The isolates P3, P6, P7, P8, P14, P 21, and P 25 from P. filamentosus showed maximum activity against the all the screened enzymes. In the case of B. bakeri, maximum activity was showed by T3, T5, T8, T20, and T23. Sixty five percentage of the isolates are capable of producing two of the three enzymes in B. bakeri, in case of P. filamentosus 84% of the isolates were able to do so.
The composition of enzyme producing bacterial flora in the fish digestive tracts are correlated to their feeding habits. Kar and Ghosh (2008) reported higher densities of proteolytic bacterial strains in carnivore bottom feeder Channa punctatus and cellulolytic strains in herbivore column feeder, Labeo rohita. Lipase producing bacteria were abundant in the guts of fishes which prey on a variety of live organisms (copepods, mysids, amphipods) rich in highly unsaturated fatty acids (Murugan et al., 2009). The occurrence of proteolytic, cellulolytic, and amylolytic bacteria in the gut has been suggested as an omnivorous feeding habit of the fish (Ghosh et al., 2010). Diverse bacterial flora with different enzyme producing activity reflects the omnivorous feeding nature of these two fishes analyzed. This result indicates that there is also a distinct microbial source of enzymes, apart from the endogenous sources in fish gastrointestinal tracts. In fish, it has been reported that Bacteroides and Clostridium sp. have contributed to the host’s nutrition, especially by supplying fatty acids and vitamins (Sakata, 1990). Depending on the diameter of clearing zone the isolates were classified as those with very good, good and poor activity (Table 2) on enzyme assay plates. Results revealed that around 15% of the isolates (Table 2) have very good amylolytic and gelatinolytic activity.
|
Table 2 Range of enzyme activity of the bacterial isolates from gut of B. bakeri and P. filamentosus Note: *> 20mm; **10-19mm; ***< 9mm; Bb – Barilius bakeri, Pf: Puntius filamentosus |
2.3 Antibacterial activity of Fish Gut bacteria
Bacterial strains with inhibitory activity have been shown to inhibit pathogenic bacteria both in vitro and in vivo through several different mechanisms. Selected bacterial isolates that showed good hydrolytic enzyme potential were further screened for their antagonistic activity against shrimp, fish and human pathogens belonging to the genera Vibrio, Aeromonas, Pseudomonas and Salmonella that generally causes diseases in fish, shrimp and human. Maximum inhibitory activity was shown by isolates T14 and T3 against Vibrio alginolyticus. None of the isolates were antagonistic against Aeromaonas spp., Salmonella spp., and Pseudomonas aeruginosa. Out of 58 isolates, 25% showed antagonism against at least one pathogenic Vibrio (Table 3). These results suggest that intestinal bacteria with antibacterial abilities may inhibit the growth of invading bacteria in intestine of fish, to some extent. The antibacterial effect of bacteria is generally due to production of antibiotics, bacteriocins, siderophores, lysozymes or proteases, and alteration of pH values by the organic acids either singly or in combination: (Gatesoupe, 1999; Balcazar et al., 2006). Though the antibacterial activity of the isolates in general was found to be less some of the isolates showed very good activity shrimp pathogens such as V. alginolyticus, V. splendidus and V. foluvialis.
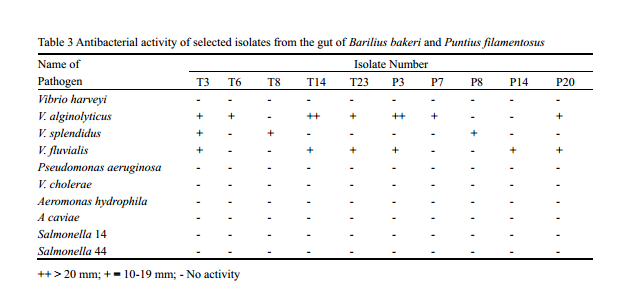 Table 3 Antibacterial activity of selected isolates from the gut of Barilius bakeri and Puntius filamentosus ++ > 20 mm; + = 10-19 mm; - No activity |
2.4. Effect of environmental parameters on growth of selected isolates
Although different bacterial genera can invade in to the gut of the fish, colonisation and survival depends on their adaptability to the microenvironment of the gut. The process of colonisation in early developing fish larvae or fry is complex and seems to be affected by bacterial load in the water (Ringø et al., 1995), which in turn a proxy of physico chemical parameter of the system. Understanding the optimum condition required for the colonisation of the isolates may help in the development of culture system and as probiotics in later stage. Optimum growth is obtained at 37℃, while temperature range preferred by majority of the isolates was in between 27℃-37℃; none of the isolates were able to grow at 4℃. Selected isolates showed growth in all range of salinity (0-25ppt) used in the experiment, with maximum growth rate at 25 ppt, showing the requirement of salt content for better growth. No growth is noted below pH 2, and optimum range favoured by most of the isolates was between 6 and 8. (Table 4).
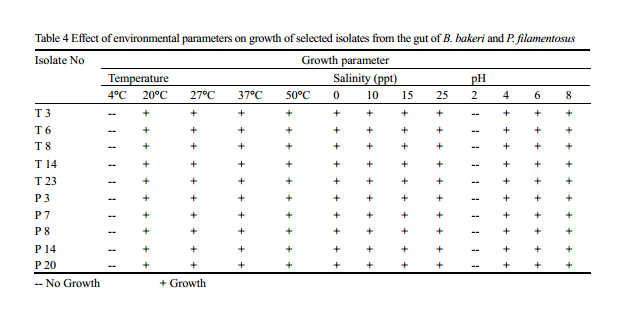 Table 4 Effect of environmental parameters on growth of selected isolates from the gut of B. bakeri and P. filamentosus -- No Growth; + Growth |
3 Conclusion
Bacteria present in the aquatic environment may influence the composition of the gut microbiota in fish and impossible to avoid them being a component of the diet (Cahill, 1990). The present study shows gut of B. bakeri and P. filamentosus were colonized by diverse genera of bacteria and are capable of producing hydrolytic enzyme at varying levels. It seems logical to think that the enzymatic mass lodged in the digestive tract might interfere in a considerable way with a major part of the metabolism of the host animal (Rasiah et al., 2009). Population levels of amylolytic strains were highest in the gut of both fishes. This can be correlated with the omnivorous feeding habit of the fishes. This also indicates that there is a distinct microbial source of digestive enzymes, such as amylase, gelatinase and lipase apart from the endogenous sources in fish gastrointestinal tracts. This information along with the antibacterial activity of the selected isolates justifies their ability as a potential probiotic in ornamental fish culture.
Acknowledgement
The authors wish to express their gratitude to Kerala State Council for Science, Technology and Environment (KSCSTE) for the financial support and Nansen Scientific Society, Bergen for the fellowship. First author is grateful to the Nansen Environmental Research Centre, India (NERCI) for the support rendered.
References
Balcazar J.L., de Blas I., Ruiz-Zarzuela I., Cunningham D., and Muzquiz J.L., 2006, The role of probiotics in aquaculture, Veterinary Microbiology, 114: 173-186
http://dx.doi.org/10.1016/j.vetmic.2006.01.009
Buchanan R.E., and Gibbns N.E., 1984, Family VI.Acetobacteraceae. In: Bergey’s Manual of Systematic Bacteriology, Vol.1 (9th ed.). Holt JG (eds). The Williams and Wilkins Co., Baltimore, pp. 267-78
Cahill M. M., 1990, Bacterial flora of fishes: a review, Microbial Ecology, 19: 2141
http://dx.doi.org/10.1007/BF02015051
Castex M., Liet C., Dominique P., Pierrette L., Nelly W., Jean L. N., Philippe S., and Catherine M., 2008, Probiotic P. acidilactici application in shrimp Litopenaeusstylirostris culture subject to vibriosis in New Caledonia, Aquaculture, 275: 182-193
http://dx.doi.org/10.1016/j.aquaculture.2008.01.011
Frazier W.C., 1926, A method for the detection of changes in gelatin due to bacteria, Journal of Infectious Diseases, 39: 302
http://dx.doi.org/10.1093/infdis/39.4.302
Gatesoupe F.J., 1999, The use of probiotics in Aquaculture, Aquaculture, 180: 147-165
http://dx.doi.org/10.1016/S0044-8486(99)00187-8
Ghosh D., eds., 2010, Probiotics and Intestinal defensins: Augmenting the First Line of Defence in Gastrointenstinal Immunity: Ln: Nair, G. B. and Takeda, Y. Probiotic foods in health and Diseases. Delhi: Oxford & IBH Publishing Co, pp 61-74
Gomez-Gil B., Roque A., and Turnbull J.F., 2000, The use and selection of probiotic bacteria for use in the culture of larval aquatic organisms, Aquaculture, 191; 259-270
http://dx.doi.org/10.1016/S0044-8486(00)00431-2
Kar N., and Ghosh K., 2008, Enzyme producing bacteria in the gastrointestinal tracts of Labeorohita (Hamilton) and Channapunctatus (Bloch), Turkish Journal of Fisheries and Aquatic Sciences, 8 (1): 115-120
Madhu K., Madhu R., and Gopakumar G. 2009, Present scenario of marine ornamental fish trade in India, Captive breeding, culture, and trade and management strategies. Fishing Chimes, 28:10-11
Monod J., 1949, The growth of bacterial cultures, Annual Review of Microbiology, 3; 371-394
http://dx.doi.org/10.1146/annurev.mi.03.100149.002103
MujeebRahiman K.M., Yousuf J., Thomas P., and Mohammed Hatha A.A. 2010, Probiotic effect of Bacillus NL 110 and Vibrio NE 17 on the survival, growth performance and immune response of Macrobrachiumrosenbergii (de Man), Aquaculture research, 41: 120-134
http://dx.doi.org/10.1111/j.1365-2109.2009.02473.x
Murugan A., Dhanya S., Sreepada R.A., Rajagopal S., and Balasubramanian T., 2009, Breeding and mass scale rearing of three spotted seahorse, Hippocampus trimaculatus, Leach under captive conditions, Aquaculture, 290: 87-96
http://dx.doi.org/10.1016/j.aquaculture.2009.01.033
Olafsen J.A., 2001, Interactions between fish larvae and bacteria in marine aquaculture, Aquaculture, 200: 223-247
http://dx.doi.org/10.1016/S0044-8486(01)00702-5
Radhakrishnan K.V., and Kurup B.M., 2006, Inland indigenous ornamental fish resources of Kerala, Fishing Chimes, 12: 27-33
Rasiah I. A., and Rehm B. H., 2009,One-step production of immobilized alpha-amylase in recombinant Escherichia coli. Applied Environmental Microbiology,75(7) 2012-2016
http://dx.doi.org/10.1128/AEM.02782-08
Ray A.K., Ghosh K., and Ringo E., 2012, Enzyme-producing bacteria isolated from fish gut: a review, Aquaculture Nutrition, 18(5): 465-492
http://dx.doi.org/10.1111/j.1365-2095.2012.00943.x
Rhodes M. E., 1959, The characterization of Pseudomonas fluorescens, Journal of general microbiology, 21:221-263
http://dx.doi.org/10.1099/00221287-21-1-221
Ringø E., Strom E., and Tabacheck J., 1995, Intestinal microflora of salmonids: a review, Aquaculture, 26: 773-789
http://dx.doi.org/10.1111/j.1365-2109.1995.tb00870.x
Ringø E., Gatesoupe F.J., 1998, Lactic acid bacteria in fish; a review, Aquaculture, 160: 177-203
http://dx.doi.org/10.1016/S0044-8486(97)00299-8
Sakata T., 1990, Microflora in the digestive tract of fish and shellfish, In, Lesel, R. (Ed.), Microbiology in Poecilotherms, Elsevier, Amsterdam, 171-176
Stickney R.R., and Shumway S.E., 1974, Occurrence of cellulase activity in the stomachs of fish, Journal of Fish Biology, 6: 779-790
http://dx.doi.org/10.1111/j.1095-8649.1974.tb05120.x
Subha G., 2013, Digestive Physiology and Role of Gastrointestinal Enzymes in Fish Gut: A Specialized Review. International Journal of Recent Biotechnology, 1 (2): 15-16
Verner-Jeffreys D.W., Shields R.J., Bricknell I.R. and Birkbeck T. H 2003, Changes in the gut-associated microflora during the development of Atlantic halibut (Hippoglossushippoglossus L.) larvae in three British hatcheries, Aquaculture, 219: 21-42
http://dx.doi.org/10.1016/S0044-8486(02)00348-4
Verschuere L., Romout G., Sorgeloos P. and Verstrate W 2000, Probiotic bacteria as biological control agents in aquaculture. Microbiology and Molecular Biology Reveiws, 64(4): 65-671
http://dx.doi.org/10.1128/MMBR.64.4.655-671.2000
Vine N.G., Leukes W.D., Kaiser H., Daya, S., Baxter, J., and Hecht, T., 2006 Competition for attachment of aquaculture candidate probiotic and pathogenic bacteria on fish intestinal mucus, Journal of Fish Diseases, 27: 319-32
. PDF(442KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Nashad M.
. Mujeeb Rahiman K.M.
. Ajin A.M.
. Mohamed Hatha A.A.
Related articles
. Probiotics
. Ornamental fish culture
. Gut microflora
. Puntius filamentosus
. Barilius bakeri
Tools
. Email to a friend
. Post a comment
.png)


