Research Article
Effects of Basil Leaf (Ocimum gratissimum) as Dietary Additives on Growth Performance and Production Economics of Clarias gariepinus 
2 Department of Aquaculture and Fisheries Management, University of Ibadan, Ibadan, Nigeria
 Author
Author  Correspondence author
Correspondence author
International Journal of Aquaculture, 2017, Vol. 7, No. 6 doi: 10.5376/ija.2017.07.0006
Received: 27 Jan., 2017 Accepted: 20 Mar., 2017 Published: 26 Apr., 2017
Adewole A.M., and Faturoti E.O., 2017, Effects of basil leaf (Ocimum gratissimum) as dietary additives on growth performance and production economics of Clarias gariepinus, International Journal of Aquaculture, 7(6): 42-50 (doi: 10.5376/ija.2017.07.0006)
Synthetic antibiotics have been widely used in fish culture systems as performance enhancers and controlling stress. These antibiotics are expensive and induce microbial resistance with consequent environmental effects. Phytogenic plants have potential as alternative antibiotics, but there is little information on their utilization in fish nutrition. Therefore the evaluation of Basil (Ocimum gratissimum) leaf meal on the growth response, nutrient utilization and production economics of Clarias gariepinus. In a 12 week feeding experiment, juveniles (n =20, weight:10.94±0.02 g) were used to assess the effect of different concentrations of Ocimum gratissimum at OGM1- OGM6; (0.00; 0.125; 0.25; 0.5; 0.10; 2.00%). A total of 120 fish were randomly allotted into treatments in triplicates, fed twice daily at 5% body weight in completely randomized design. Data for the growth and nutrient utilization parameters such as: Total weight gain (TWG), Total final weight (TFW); Mean weight Gain (MWG), specific growth rate (SGR), feed conversion ratio (FCR); and economic indices such as profit indices, (PI), were determined. Data were analysed using descriptive statistics and ANOVA at α 0.05. The highest TWG and FWG (916.67 ±17.98 g; 698.83±17.54 g) was from the fish fed OGM2 diet and the least (137.92±2.97 g; -81.08±2.97 g) from fish fed OGM6 diet. While the highest MWG was from fish fed OG3 diet and the least was from OG6 diet. The fish fed OGM3 had the highest PI (5.72) and the least (1.53) was from fish fed OGM6 diet. Therefore adoption of Ocimum gratissimum meal diet at the established dosages of OGM2 and OGM3 in sustainable catfish production in Africa is being advocated based on its availability and profitability.
1 Introduction
Nigeria has a varieties of medicinal herbs spread over the country due to favorable weather condition. All these herbs possess a number of chemical substances that facilitate their utilization in the treatment of poultry diseases, poultry nutrition and help in reduction of cost in poultry (Akhtar et al., 1984; Nworgu et al., 2013) and in fish (Adewole, 2014; Adewole, 2015). Additionally, most of these herbs have been known to have different arrays of secondary metabolites, which gives them the unique properties of being used for various: medicinal and pharmacological activities such as: antioxidant, antiparasitics and antimicrobial (Hamilton–Miller, 1995). Furthermore, continuous research into plant bioactive materials known as phytogenic which serves as good alternative to synthetic antibiotics which their utilization has led to the problem of microbial drug resistance and residue in tissues of food animals (Taylor et al., 2010). Therefore, the need for animal / fish nutritionists to look inward for alternative (Wei and Shibamato, 2007; Adewole, 2014; Adewole, 2015). Such alternatives feed additives to synthetics are probiotics, prebiotics, organic acids, enzymes and phytogenics (Windisch et al., 2007; Ndelekwute et al., 2014; Adewole and Awosusi, 2015). Herbs (extracts and essential oils) fall into the class of phytogenic compounds, being presently explored for use as feed additives and growth enhancers (Windisch et al., 2007; Bello et al., 2012; Odoemelam et al., 2013). Some of these herbs are indigenous to Africa; they include garlic (Allium sativum), bitter leaf (Vernonia amygdalina) and scent leaf (Ocimum gratissimum) among others (Osuji et al., 1995).
Ocimum gratissimum also called basil leaf is an aromatic, herbaceous and perennial plant. Its vernacular name includes nchanwu, daidoya and effrin in Igbo, Hausa and Yoruba languages respectively in Nigeria (Epharim et al., 2000). It’s popular among users due to its antimicrobial properties (Orafidiya et al., 2000). The objectives of this study are to evaluate the effect of graded levels or dosages of Ocimum gratissium on growth performance, nutrient utilization and production economics of juvenile catfish in sustainable aquaculture development in Nigeria.
2 Materials and Methods
Study area
The study was conducted in the Department of Aquaculture and Fisheries Management Postgraduate’s Teaching and Research Laboratory, University of Ibadan, Ibadan, Nigeria.
Collection, identification, preparation and processing of the plants materials
Identification of the plant material was done in the Department of Forestry and Renewable Resources, Faculty of Agriculture and Forestry Resources, University of Ibadan, Ibadan Nigeria. The collection, preparation, processing and preservation were done practically as reported by Igbakin and Oloyede (2008) and Ogbuewu et al. (2010).
Calculated dosages for basil leaf (Ocimum gratissimum)
There were several attempts to evaluate the pharmacological dosage of O. gratissimum in recent time as reported by Ojo et al. (2013) and Adedosu et al. (2012) with the dosage ranging from 100 – 400 mg/kg body weight. Also a higher dosage had been reported by Fandouhan et al. (2008) at 1500 mg/kg in Wistar rats. The dosage values of basil leaf (Ocimum gratissimum) used in this study was based on the extrapolated values of 0.5 g/kg in rats reported by (Ibironke and Ekpo, 1992). The final dosages ranged from 0.0125 – 2.0 g/100g body weight.
Formulation and preparation of experimental diets
The various ingredients used in compounding the diets were brought from Adom Feedmill and Veterinary Supply Shop at Orogun, and Bodija Market, Ibadan, Oyo State Nigeria. The experimental diets were formulated using algebraic method along with least cost formulae of Falayi (2003).The diets were prepared using the various calculated dosages to produce Ocimum gratissimum meal diets and coded as follows: OGM1 (control), OGM2, OGM3, OGM4, OGM5 and OGM6 diets respectively as shown in Table 1. The fish feed were prepared following the methods of Adewole and Awosusi (2015).
|
Table 1 Percentage composition of ingredients (g/100g diets) in Ocimum gratissimum meal diets for feeding trials Note: OGM = Ocimum gratissimum Meal |
Stocking, feeding and sampling of experimental fish
A total of 120 fingerlings of Clarias gariepinus of mean weight of (10.94±0.01 g) were acclimatized for 21 days in holding tanks in the departmental laboratory. They were fed with commercial feed bought from Durantee feeds. After acclimatization, the fingerlings were randomly allocated into the experimental tanks in triplicates, using completely randomized design.
The fish were starved for 24 hours before the commencement of the feeding trial, and not fed on the weighing day as recommended (Kumar et al., 2010a). The fish were fed at 5% body weight, twice daily between 8.00 am and 19.00 pm for 12 weeks. The weight of the fingerling in each tank was measured using a Scout Pro Weighting balance SP x 402 of 400 g maximum loading and 0.01 g resolution (OHAUS Corp. Pine Brook, NJ, USA).The new weight attained was done through batch weighing to get the weight), while, five fingerlings were taken randomly from each tank at fortnight to measure the growth rates in terms of total length attained (The fish length measurement was taken using 1 m fish measuring board of 0.01 cm calibration).The fish were monitored for mortality daily.
Growth performance, nutrient utilization, production cost and economic evaluation of feeding trials of Clarias gariepinus
At the end of the culture period the rearing indices such as the growth rates: mean weight gain, specific growth rate, relative growth rate, and nutrient utilization parameters such as total feed intake, feed conversion ratio, protein intake, protein efficiency ratio, condition factor, and survival rate were computed and analyzed as reported by (Adewole and Awosusi, 2015; Adewole, 2015).
The growth was expressed as Mean Weight Gain (MWG) according to (Adewole, 2015)
.png)
Where:
W0 = initial mean weight
W1= final mean weight
n= number of experimental weeks
Specific Growth Rate (SGR) according to (Adewole and Awosusi, 2015)
.png)
Where:
Ln = Natural log
W0 = initial mean weight
W1= final mean weight
T = time interval
Relative Growth Rate (RGR) according to (Ajayi et al., 2013)
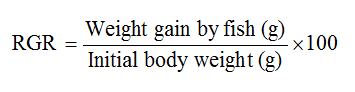
Condition Factor (K) according to (Ajayi et al., 2013)
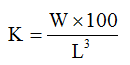
Where:
W = final weight
L = Final standard length
Feed Conversion Ratio (FCR) according to (Adewole and Awosusi, 2015)
.png)
Total Feed Intake (TFI) according to (Oyelese, 2007)
TFI = Sum of the amount of feed fed the fish per week
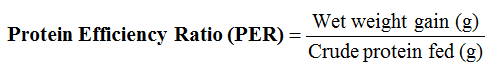
Protein Intake (PI) = Feed intake x % Protein in diet
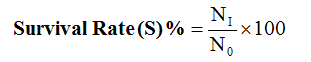
Where:
NI = final number of fish at the end of experiment
N0 = initial number of fish at the beginning of experiment
The production cost in Naira of the experimental diets were calculated following the method of Faturoti and Lawal (1986) and Mazid et al. (1997) based on the current market prices of the ingredients used for formulating the diets.
Statistical Analysis
Data collected were subjected to Analysis of Variance (ANOVA) at α 0.05. Comparisons among treatment means were separated using Duncan’s Multiple Range Test to determine the level of significance based on Statistical Analysis System (SAS, 2008).
3 Results and Discussion
The total final weight attained ranged from 137.92 ± 2.97 g to 916.67 ± 17.98 g. The highest total final weight value was from OGM2 diet, followed closely by 869.25 ± 7.99 g from OGM3 diet, while the lowest was from the fish fed OGM6 diet. The fish fed the OGM2 andOGM3 diets had significantly higher (p<0.05) total final weight than fish fed OGM4 andOGM1 diets which were also significantly different (p<0.05) from the fish fed OGM5 andOGM6 diets respectively (Table 2).
|
Table 2 Growth performance of Clarias gariepinus fed Occimum gratissimum additive meal for 84 days
Note: Data mean values with different superscripts in each row are significantly different (P>0.05), while without data are insignificantly different P>0.05
|
Mean weight gain ranged from -6.22 ± 0.24 g to 40.69 ± 0.49 g, with the highest value was from OGM3 diet and the lowest value was from fish fed OGM6 diets. The fish fed OGM2, OGM3, OGM4 diets had significantly higher (p<0.05) mean weight gain than fish fed OGM1 diet, which was significantly different (p<0.05) from the fish fed OGM5 and OGM6 diets respectively. Specific growth rate ranged from -0.24±0.01 (%/day) to 0.71 ± 0.00 (%/day). The highest value was from fish fed OGM3 diet, while the lowest value was from fish fed OGM6 diet. The fish fed OGM2, OGM3, OGM4 diets had significantly higher (p<0.05) specific growth rate than OGM1 diet, which was also significantly different (p<0.05) from the fish fed OGM5, OGM6 diets respectively (Table 2).
Fish productivity index ranged from -35.87±1.36 to 1876.42±28.07, the highest value is from fish fed OGM2 diet and the lowest value was from the fish fedOGM6 diet. The fish fed OGM2, OGM3, OGM4 diets had significantly higher (p<0.05) fish productivity index than the fish fed OGM1 diet, which was significantly different (p<0.05) from the fish fed OGM5, OGM6 diets respectively (Table 2). The final condition factor ranged from 0.48 ± 0.04 to 0.69±0.03. The highest final condition factor value was from the control OGM1 diet, followed closely by 0.67±0.06 from OGM3 diet, while the lowest value was from OGM6 diet respectively. The fish fed the OGM1, OGM3 diets had significantly higher (p<0.05) final condition factor than OGM2, OGM4 diets, which were significantly different from OGM5, OGM6 diets. The percentage survival ranged from 65.00% to 90.00%. The highest value was jointly from OGM2 and OGM4 diets, while the lowest percentage survival was from fish fed OGM6 diet as shown in (Table 2).
Total feed intake ranged from 1701.18±27.69 g to 2344.01±35.74 g within the treatments, the highest value was from OGM3 diet and the lowest value was from the OGM6 diet. The fish fed the OGM2, OGM3 diets had significantly higher (p<0.05) total feed intake than the OGM4, OGM1 diets, which were significantly different from OGM5 and OGM6 diets. Total protein intakes ranged from 707.35±11.51 to 940.70±12.22 g within the treatments, and follow the same trend as total feed intake (Table 2). The feed conversion ratio values ranged from 4.44±0.07 to 18.08±0.57 across the treatments. The highest value was from the fish fed the OGM5 diet and the lowest was from OGM3 diet. The fish fed the OGM5 diet had significantly higher (p<0.05) feed conversion ratio than OGM6, which was significantly different from other diets (Table 2). Protein efficiency rate values ranged from -0.17±0.01 to 0.75±0.02 within the treatments. The highest value was from fish fed OGM3 diet and the lowest was from fish fed OGM6 diet. The fish fed OGM6, OGM5, OGM4 diets had significantly higher (p<0.05) protein efficiency rate than OGM3 which was significantly different from the OGM2 and OGM1 diets respectively (Table 2).
The result of the production cost of experimental diet of Ocimum gratissimum (OGM) meals (Table 3) showed that the highest profit index 5.72 was from OGM3 diet and the least 1.53 was from OGM6 diet. The highest net profit was 114.97 was from OGM2, followed closely by 94.46 from OGM3 and the least value of -273.86 was from OGM6 diet.
|
Table 3 Production Economics of Ocimium gratissimum meal experimental diet Note: Mean are values of three replicates, N = Naira |
4 Discussions
The fact that weight gain was not reported in all the treatments was an indication that the fish did not responded positively and effectively to all the diets. The higher inclusions of the diet ≥ OGM5 had gross consequence on the growth and energy supply of Clarias gariepinus. This is contrary to the reported adequate provision for growth and dietary energy supply of catfish (Fagbenro and Arowosoge, 1991). Furthermore, it also implies that the experimental diets contained anti – growth factors or that Clarias gariepinus was highly sensitive to the active components of the plants at higher inclusions.
The chemical composition of the essential of O. gratissimum can change accordingly with geographical distribution and daytime of collection. Vincenzi et al. (2000) reported that estragole a naturally occurring genotoxic carcinogen in experimental animals after chronic exposure or after a few repeated doses can be present in O. gratissimum. The reduced total final weight gain, mean weight gain, protein efficiency ratio, feed conversion ratio and specific growth rate, relative growth rate in tested C. gariepinus at ≥ OGM5 might not be unconnected with the presence of this caricnogenic estragole in the O. gratissimum used in the present study. Although the chemical components of the herb O. gratissimum was not determined to sanctify its usage in phytomedicines.
Furthermore, the reduction in live body weight of C. gariepinus beyond ≥ OGM5 were similar to Ogbuewu et al. (2010) who reported similar reduction in the live weight of rabbit beyond 5% neem leaf meal (NLM) diet, which implied a reduction in growth rate. It appears that these neem bioactive compounds are responsible for depression in nutrient utilization and growth in rabbits. Similarly, the leaves of O. gratissimum have been repeated to contain saponins. The effects of saponins might have contributed to the clinical signs of loss of appetite (as deduced by reduction in feed intake) and loss of weight observed in all rabbits administered O. gratissimum extract Ephraim et al. (2000).
Saponins are known to be toxic to body systems (Watt and Breyer-Brandwrijk, 1962; Edeoga et al., 2006). Opdyke (1974) further reported that it is likely that the toxic potential of the O. gratissimum oil is due to thymol, which is the major component with a P.O. LD of 3.75-5.67 g/kg weight body weight. The feed intake, mean feed intake, total protein intake and mean protein intake varied significantly within the group of fish fed OGM’s diet when compared with the control. There were reduction in all these parameters in fish fed ≥ OGM5 diets; this might be due to higher concentration of saponin at the higher inclusion levels, as observed by Effraim et al. (2000).
The higher voluntary feed intake at ≥ OGM5 in the C. gariepinus were also similar to the reported increase in the total quantities of tested neem leaf meal consumed by rabbit, which increased with the increasing concentration of the neem leaf (T1 (0.0g), T2 (234.98 g) T3 (665.06 g) and T4 (1237.60 g)) respectively, but with attendant reduction in body weight as the concentration of neem leaf meal increased.
The feed striking time and feed acceptability indices may not be significantly different (p>0.05) at the end of feeding trial in both the fish fed OGM’s tested group and the control diet, however, the numerical variation observed for these parameters could be due to the varying levels of O. gratissimum leaf in the diets affecting the palatability. The voluntary feed intake which is a function of acceptability, palatability and utilization (Mc Donald et al., 1998), also showed significant difference (p>0.05) within the treatments which inferred that the inclusion of OGM’s do negate the response of the fish to the diet.
This result is contrary to the report of Eyo and Ezechie (2004) when they feed “Heteroclarias” fingerlings with rubber feed meals. They reported that acceptability and striking time of the experimental diets by the fish were better in the control diet compared to those with rubber seed. Gwiazda et al. (1983) had reported that feed deterrent, depressant reduced palatability and acceptability index. The findings from the present study implies that acceptability index is not better in the experimental diets than the control, but voluntary intake, nutritional efficiency, feed utilization were similar to the observation of Devakumar and Dev (1993). The feed conversion ratio from OGM1-OGM4 indicates better utilization by the C. gariepinus and is within the range of 2.56 - 6.5 reported by Adewole (2015), but for the ≥ OGM5 diets, it indicates poor utilization of these diets. The increasing percentage mortality as the concentration increases or ≥ OGM5 diets showed in this present study is in agreement with Devakumar and Dev (1993), which reported as high as 50% mortality in livestock fed neem cake. The highest percentage mortality was observed from the fish fed OGM6 diet. This may be due to the presence of antinutritional and highly toxic compounds such as saponins, thymol, tannins, glycosides and alkaloids that are known to be toxic to body system Ojo et al. (2013). Furthermore, the observed mortality in C. gariepinus fed ≥ OGM5 diets may be due to the influence of these bioactive components of O. gratissimum interfering with protein metabolism at higher concentrations as reported by Iweala and Obidoa (2010) that the consumption of O. gratissimum significantly reduced the serum protein in rats. The quantification of these bioactive substances in O. gratissimum and their pharmacological impacts were evaluated in the present study. Effraim et al. (2000) reported that O. gratissimum extract contain flavonoids, while Nyahangare et al. (2012) observed that ordinarily, these flavonoids are umbiquitous in plants are not toxic. However, their occurrence in plant at such high levels coupled with other bioactive compounds and high doses may enhance the apparent toxicity of O. gratissimum in the present study as reflected in reduced feed intake and higher mortality experienced in C. gariepinus fed ≥ OGM5 diets compared to the other diets, with increased feed intake and reduced mortality. The above submission is similar to Ojo et al. (2013) that indicated the toxic effect of O. gratissimum leave extracts at 4 µg/kg may be due to combined toxicity of the phyto - chemical constituents from the leaves of O. gratissimum. The mortality observed in the control and OGM2-OGM4 diets may be attributed to the handling stress during the culture period. Contrarily, Iweala and Obidoa (2010) reported the positive influence of O. gratissimum on the weight of Swiss albino rat fed 5% of ground leaves of O. gratissimum for 6 months compared to the control.
It was observed that Ocimum gratissimum supplemented diet resulted in increased weight gain in the animals which was attributed to the nutrient composition of leaves of O. gratissimum. These elaborate nutrients that can increase weight includes: carbohydrates, lipids, protein, minerals and vitamins (Edeoga et al., 2006). While Oladunmoye (2006), reported that O. gratissimum serve as immune booster by increasing the weights of both rats infected with Escherichia coli and treated with ethanolic extract of O. gratissimum and rats given O. gratissimum alone. The author conclusively recommended that low concentration or regular intake of the plant extract is able to cure E.coli infection and increase the weight too. Therefore the results obtained for C. gariepinus fed OGM2-OGM4 diets were similar to Vincenzi et al. (2000), Iweala and Obidoa (2010) and Oladunmoye (2006).
Economic evaluation of feeding C. gariepinus fingerlings on Ocimum gratissimum diets, indicated that the lower inclusion levels (OGM2-OGM4) had the cost of production and benefit cost positively favoring their utilization, since these values are greater (>1 .0), while the upper inclusions (OGM5-OGM6) had cost benefit ratio of less than (<1.0) and also lower than the control. This result obtained here similar to the reported profitability of Roselle and Bitter leaf evaluated for the same fish Adewole (2014); Adewole (2015) respectively. Furthermore, these observations may be due to fact that the herbs being used as spices, could be better tolerated at lower inclusion levels, because spices or additives have some chemical compounds that may be harmful to animal (fish) especially if used in excess of recommendation Odo (2007). The result from this finding revealed significantly better total final weight, mean final weight, and better feed intake at the lower inclusion levels, suggesting more flesh or muscle production and higher income to the farmers. The result obtained also support the verdict of Crampton (1985) that reduced feeding and good conversion efficiencies positively affected the profitability of an intensive aquaculture system. The best cost benefit ratio was observed in OGM2 diet with the highest total feed intake and total final weight gain. Therefore, the inclusion of basil leaf at 0.125% is recommended for the sustainable production of catfish in Nigeria / Africa, while at higher dosage of 1% and above it could indicate toxic effects.
Adedosu O.T., Badmus J.A., Afolabi O.K., and Yakubu F.O., 2012, Effect of methanolic leaf extract of Ocimum gratissimum (Linn) leaves on sodium arsenite-induced toxicity in rats, Journal of Pharmacology and Toxicology, 7:259-266
https://doi.org/10.3923/jpt.2012.259.266
Adewole A.M., 2014, Effects of Rosselle as dietary additives on growth performance and production economics of Clarias gariepinus, Journal of Emerging Trends in Engineering and Applied Sciences (JETEAS), 5 (7): 1-8
Adewole A.M., 2015, Production economics of Bitter leaf (Vernonia amygdalina) as dietary additive fed to Clarias gariepinus, Journal of Emerging Trends in Engineering and Applied Sciences (JETEAS), 6(7): 267- 275
Adewole A.M. and Awosusi A.O., 2015, Growth performance, carcass yield and organosomatic indices of Clarias gariepinus fed propolis and ag-zyme as dietary additives, African Journal of Education, Science and Technology, 1(4), pp16-20
Akhtar M.S., Afzal H., and Chauddry F., 1984, Preliminary in-vitro antibacterial screening of Bakain and Zarisk against Salmonella, Medicase, 9: 6-7
Ajayi I.A., Olaifa F.E., and Raimi A.A., 2013, Evaluation of nutritional and toxicological effects of Treculia africana (Decne.) seed flour – supplemented diets on Clarias gariepinus (African catfish) fingerlings, Food science and Quality Management vol, 17, 62-70
Bello O.S., Emikpe B.O., and Olaifa F.E., 2012, The body weight changes and gut morphometry of Clarias gariepinus juveniles on feed supplemented with walnut (Tetracarpidium conophorum) and Onion (Allium cepa) bulb residues, Int. J. Morphol, 30(1):253-257
https://doi.org/10.4067/S0717-95022012000100045
Crampton V.O., 1985, The application of nutritional findings of the formulation of practical diet, In: Nutrition and feeding in fish, Academic Press, London, Pp 447
Devakumar X.C., and Dev S., 1993, Chemistry Society, Pest Science Neem Research Development Publication, No 3 India, 63-96
Edeoga H.O., Omosun G., and Uche L.C., 2006, Chemical composition of Hyptis suaveolens and Ocimum gratissium hybrids from Nigeria, Afr. J. Biotechnology., 5: 892-895
Ephraim K.D., Salami H.A., and Osewa T.S., 2000, The effect of aqueous leaf extract of Ocimum gratissium on hematological and biochemical parameters in rabbits, Afr. J. Biomed. Res., 3: 175-179
Eyo J.E. and Ezechie C.U., 2004, The effect of rubber (Havea brasiliensis) seed meal based on diet acceptability and growth performance of Heterobranchus bidorsalis and Clarias gariepinus hybrid, Journal of Agricultural Research, 10:20-25
Fagbenro A.O. and Arowosoge I.A., 1991, Utilization of agricultural waste and by product in fish feeds production in Nigeria, Proc. 6th Ann. FISON Conf. Lagos.1991, 121-130
Falayi B.A., 2003, Techniques in fish feed manufacture, In: Proceeding of the joint Fisheries Society of Nigeria / National Institute for Freshwater Fisheries Research/FAO – National Special Programme for Food Security, National workshop on Fish feed development and feeding parties in Aquaculture, (Eyo, A. A. Ed.) held at National Institute for Fresh Water Fisheries Research, New – Bussa 19th September 2003
Fandohan P., Gnonlonfin B., Laleye A., Gbenou J.D., Darboux R., and Moudachirou M., 2008, Toxicity and gastric tolerance of essential oils from Cymboppogon citratus, Ocimum gratissimu, and Ocimum basilicum in wistar rats, Food Chem Toxicol, 46(7): 2493-7
https://doi.org/10.1016/j.fct.2008.04.006
PMid:18511170
Faturoti E.O. and Lawal L.A., 1986, Performance of supplementary feeding and organic manuring on the production of Oreochromis niloticus, Journal of West Africa Fisheries, 1: 25 – 32
Gwiazda S., Noguchi A., Kitamura S., and Saio K., 1983, Effect of chlorogenic acid removal from leaf protein concentrate on fish feeding, Agricultural Biological Chemistry, 47 (3), 623-625
https://doi.org/10.1080/00021369.1983.10865694
https://doi.org/10.1271/bbb1961.47.623
Hamilton – Miller J.M.T., 1995, Antimicrobial properties of tea, Antimicrobial Agents and Chemotherapy, 39 (11) 2375-2377
https://doi.org/10.1128/AAC.39.11.2375
PMid:8585711 PMCid:PMC162950
Ibironke G.F. and Ekpo H.E., 1992, Hypoglycemic activity of Occimum gratissimum in normal and alloxan diabetic rabbits, Afr. J. Biomed. Res., 161-164
Igbakin A.P. and Oloyede O.B., 2008, Biochemical comparison of the antidiabetic properties of the ethanolic and normal saline extracts of the leaves of Ocimum gratissium in diabetic rat, Science Research Annals, Vol 4 No 9, pp 49 – 58
Iweala E.E.J. and Obidoa O., 2010, Studies on some biochemical and histological changes assovated with long term consumption of leaves of Ocimum gratissimum L. in male rats, American Journal of Food Technology, 5 (6): 376-384
https://doi.org/10.3923/ajft.2010.376.384
Kumar V., Harinder P.S.M., Werner A., and Becker K., 2010, Physological, haematological and histopathological responses in common carp (Cyprinus carpio) fingerlings fed with different detoxicified Jatropha curcas kernel meal, Food and Chemical Toxicology, 48, 2063 – 2072
https://doi.org/10.1016/j.fct.2010.05.007
PMid:20457206
Mazid M.A., Zaher M., Begum N.N., Aliu M.Z., Nehaw F., 1997, Formulation of cost – effective feeds from locally available ingredients for carp polyculture system for increase production, Aquaculture, 151:71-78
https://doi.org/10.1016/S0044-8486(96)01504-9
Ndelekwute E.K., Uzegbu H.O., Amaefule K.U., Okereke C.O., and Umoh B.I., 2014, Effect of organic acids fed through drinking water on carcass and internal organs of broiler chickens, Nig. J. Anim. Prod. Abuja, Nigeria, Pp 731-733
Nyahangare E.T., Hove Mvumi T.B.M., Hamudikuwanda H., Belmain S.R., Madzimure J., and Stevenson P.C., 2012, Acute mammalian toxicity of four pesticidial plants, Journal of Medicinal Plants Research, Vol. 6(13), pp. 2674-2680
Odo B.I., 2007, Effects of commercial feed additives on the growth performance of broilers, International Journal of Food and Agricultural Research, Vol. 4, No 1 and 2.200-205
Odoemelam V.U., Etuk I.F., Ndelekwute E.K., Iwuji T.C., Ekwe C.C., 2013, Herbs and Spices: option for sustainable animal production, J. Biol. Agric.and Healthcare, 3 (7):116-120
Ogbuewu I.P., Ucheagbu M.C., Okoli I.F., and Iloeje M.C., 2010, Toxicological effects of neem leaf meal of ethnomedicinal plant –Neem – on serum biochemistry of crossbred New Zealand white type rabbit bucks, Reports and Opinion, 2(2) 54-57
Ojo O.A., Oloyede O.I., Olarenwaju O.I., Ojo A.B., Ajiboye B.O., and Onikanni S.A., 2013, Toxicity studies of the crude aqueous leaves extracts of Ocimum gratissimum in albino rats, Journal of Environmental Sciences, Toxicology and Food Technology, Volume 6, Issue 4(Sep-Oct, 2013), PP34-39
Oladumoye M.K., 2006, Immunostimulatory activity of ethanolic leaf extract from Ocimum gratissimum in albino rat orogastrically dosed with Escherichia coli (NCIB 86), Journal of Pharmacology and Toxicology, 1 (4): 389-394
https://doi.org/10.3923/jpt.2006.389.394
Opdyke D.L.J., 1974, Thyme oil, red, Food Cosmet Toxicol, 12: 1003-1004
Orafidiya O.O., Elujoba A.A., Iwalewa F.O., and Okeke I.N., 2000, Evaluation of antidiarrhoea properties of Ocimum gratissimum volatile oil and its activity against enteroaggregative Escherichia coli, Pharm Pharmacol. Lett., 10:9-12
Osuji P., Fermandez-Riviera O.S., and Odenyo A., 1995, Improving fibre utilization and protein supply, In: Animal fed poor quality roughage, ILRI Nutrition Research and planning, Wallace R.J. and Lahlon –Kassi, A.,(eds.), Vol. 1 International Livestock Research Institutes, Addis Ababa., Pp 1-22
Oyelese A.O., 2007, Utilization of processed soyabean seeds in the diets of Clarias gariepinus fingerlings, The Nigerian journal of Forestry, vol.37, (1and 2), 112-118
SAS (Statistical Analysis System), 2008, SAS for Window, Release 9.1, SAS Institutes, Inc., Cary
Taylor D.J., Green N.P.O., and Stout G.W., 2010, Antibiotics, In: Biological Science 1&2, Third Edition Edited by Soper, R. Cambrdge University Press, The Edinburgh Building, Cambridge CB2 8RU, Uk, p518-520
Vincenzi M., Silano M., Maialetti F., and Scazzochio B., 2000, Constituents of aromatic plants: II Estragole, Fitoterapia, 71: 725-729
https://doi.org/10.1016/S0367-326X(99)00150-1
https://doi.org/10.1016/S0367-326X(00)00153-2
Watt J.M. and Breyer-Brandiwijk M.J., 1962, The medicinal and poisonous plants of Southern and Eastern Africa 2nd Edition, E and S Livingstone, Edinburgh, p1425
Wei A. and Shibamato T., 2007, Antioxidant activities and volatile constituents of various essential oils, J. Agric and Food Chem, 55 (17): 37-42
https://doi.org/10.1021/jf062959x
Windisch W., Schedle K., Plitzner C., and Kroismayr A., 2007, Use of phytogenic products as feed additives for swine and poultry, J. Anim. Sci., 86:140-148
https://doi.org/10.2527/jas.2007-0459
PMid:18073277
. PDF(305KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. A.M. Adewole
. E.O. Faturoti
Related articles
. Aquaculture
. Herbal medicine
. Catfish
. Growth promotant and dosages
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)


