2 Ethiopian Agricultural Research Organization (EARO), Fishery and Aquatic life research center, Sebeta, Ethiopia
 Author
Author  Correspondence author
Correspondence author
International Journal of Aquaculture, 2017, Vol. 7, No. 20 doi: 10.5376/ija.2017.07.0020
Received: 20 Sep., 2017 Accepted: 17 Oct., 2017 Published: 24 Nov., 2017
Urga K.T., Prabhadevi L., and Tedesse Z., 2017, Diversity and biology of fishes in the river Debbis, Ethiopia, International Journal of Aquaculture, 7(20): 126-134 (doi: 10.5376/ija.2017.07.0020)
This study on the longitudinal distribution pattern of fish fauna and fishing activities on Debbis River was carried out during January to March, 2017. Fishes were sampled by using electro fishing and data on the total length and weight were measured. The cyprinids, Garra quadrimaculata, Garra chebera, Varicorihinus beso, Labeobarbus forskalii and Labeobarbus intermedius were observed. The species Garra quadrimaculata and Garra chebera are the most dominant species followed by Varicorhinus beso. Shannon diversity index (H’) ranged from 1.05 to 1.52. Condition factor of the dominant species indicated that the fishes were in good condition with the mean value > 1.00. The highest condition factor value was that of Varicorhinus beso and Garra chebera from site two (Deb2). Males of Varicorhinus beso and females of Garra chebera were found in better condition. The gut content revealed that the fishes are omnivores. Labeobarbus forskalii, Varicorhinus beso and Labeobarbus intermedius are commercially important and the price is influenced by marketing site, season, size of fish and demand. Fishing is mainly artisanal using cast net, hook and poisoning with the seed of Millettia ferruginea during dry season.
1 Background
Inland fisheries are particularly important for the food security of poor people, as most inland fish production goes for subsistence or local consumption (FAO, 2004). In Ethiopia, most of the fish catch comes from the lakes and reservoirs (85%) and the rest comes mainly from the rivers (MOARD, 2009). Ethiopia has a rich diversity of ichthyo-fauna in its lakes, rivers and reservoirs, although they are poorly of known (Ameha and Assefa, 2004). About 94 species of fishes has described in Ethiopia (Tedla, 1973). Of the drainage systems, White Nile basin accommodates the highest diversity of fish fauna (Tesfaye and Wolff, 2014). The structure of a fish community is determined by the species present, their relative abundances, their life stages and size distributions, and their distributions in space and time (Meador et al., 1993). According to Golubtsov and Mina (2003), the total number of valid fish species known from Ethiopian waters was between 168 and 183 including 37-57 countrywide endemics. Getahun (2005a; b; c) reported 38 species and two sub-species endemic to Ethiopia. Currently, it is estimated that over 200 species of fish and numerous other aquatic resources occur in Ethiopian drainage systems (Habteselassie, 2012). Riverine fishing activities are concentrated on two of the rivers, Baro near Gambella western part of the country and the Omo in the southern area near the border with Kenya (Abegaz et al., 2010). Riverine fishery is not developed due to lack of access to suitable fishing grounds and the culture of most communities (Tigabu and Abegaz, 2007) and hence many of the drainage basins are not exhaustively explored (Getahun, 2002). The Rivers Didessa and Gudder, drains to the southwestern parts of Ethiopia are the tributaries of Blue Nile (Abay) basin. Debbis River is one of the tributaries of river Gudder which has not been explored for the fish population potential and socio-economic benefits due to difficult accessibility, security and harsh geographical features of the area. Therefore, the present study has been conducted to collect a baseline data on the diversity fish fauna, food habits, fishing and marketing activities along the course of River Debbis.
2 Results and Discussion
2.1 Numerical abundance and species composition
All the fishes collected belong to family Cyprinidae. The predominance of cyprinids was earlier reported from head of Blue Nile River (de Graaf, 2003; Oumer et al., 2011). The fish fauna of Debbis River is a mixture of Nilo Sudanic (Labeo forskalii), East African highland (Labeobarbus intermedius, Garra chebera and G.quadrimaculata) and an endemic (Varichorhinus beso) species. A total of 2389 fishes weighing 23.8 kg were caught. The total number of fishes collected was 941,764 and 684 respectively from the stations Deb1, Deb2 and Deb3 (Table 1). The total length of the fishes ranged from 5 to 33 cm for Varichorhinus beso, 4.4 to 13.6 cm for Garra chebera, 3.9 to 12.1 cm for G.quadrimaculata, 23 to 37 cm for Labeo forskalii and 21.4 to 32.8 cm for Labeobarbus intermedius. The total weight ranged from 1.7 to 306 gm for V. beso, 1.3 to 28.8 for G. chebera, 1 to 20.3 for G. quadrimaculata, 167.4 to 415 for Labeo forskalii and 109 to 415 grams for Labeobarbus intermedius.
|
Table 1 Number and species composition of fishes |
Garra quadrimaculata was the most dominant fish species (37.14%), followed by G.chebera (34.37%) and V. beso (22.05%) while Labeobarbus intermedius and Labeo forskalii were least the abundant, 3.46% and 2.58% respectively.
All the five species were collected from Deb3, however V. beso, G. quadrrimaculata and G. chebera are common to all sites. The two species, L. forskalii and L. intermedius were collected only from Deb3 (Table 1). The number of specimens caught was higher for Deb1 with (941) and lower at Deb3 (684). The Shannon diversity index was higher (H’=1.52) at Deb3 and lower (H’=1.053) at Deb1 (Table 2).
|
Table 2 Shannon diversity index (H’) at the sampling sites |
From the head of Blue Nile River Oumer et al. (2011) reported the occurrence of 17 species while Tedlo Awoke (2015) observed 8 species from Lake Tana. It was generally accepted that the low species diversity of fishes may be related to the flow variability which has an effect on fish assemblage and productivity of rivers as high flows could destroy fish habitat and wash away the already laid fish eggs. The presence of few fish species and dominance of few families in this study seemed that cyprinid fishes being riverine in origin are specifically segregated or adapted in the Blue Nile and its tributaries. Biodiversity patterns are directly and indirectly influenced by the geomorphology of riverine landscapes, which may be perceived as a nested hierarchy (Ward, 1998). The numbers of fish species in Debbis River appears to be negatively correlated with altitude. The increase in number of fish species from Deb1 to Deb3 sampling sites coincide with decline in elevation. The elevation with each site Deb1, Deb2 and Deb3 were (1919, 1902 and 1886) m.a.s.l respectively. The main pattern documented in this study was the occurrence of a distinct headwater fauna, and a sequential downstream shift in species composition. The decrease in number of fish species from lower to upper reaches were consistent with the studies carried out in other areas by Nikolsky (1963) and Golubtsov and Mina (2003). The increase in species number from upstream sites to downstream sites was associated with change in catchment area, canopy closure, substrate type, and distance from source, depth and width of rivers (Toham and Teugels, 1998). The deep habitats at the downstream site probably favor fish species richness because they potentially allow the coexistence of numerous fish species, as suggested by Sheldon (1968). These variables reflect longitudinal gradient in the study area. Width of river was the most important variable that coincided with increase in species number from Deb1 to Deb3 sites. A total of five (5) fish species found in Deb3 with its mean river width of 57.5 ± 3.74 m while the lowest number three (3) species at Deb1 and Deb2 sampling site with mean river width of 31 ± 3.37 m and 50.25 ± 4.27 m respectively. This result is conformity with Toham and Teugels (1998) in Cameroon, EDDS (1993) in India and in Ethiopia (Berie, 2007) in Beles and Gilgel Beles Rivers that a significant relationship exists between species number and width of the river. In addition, canopy closure and diversity of substrate type (sand, gravel and large rocks) were also most probable environmental gradient explaining the spatial distribution of species in the sampling sites. In present study, in Deb3 has thick vegetarian cover and diverse types of substratum than the others. Thus, the presence of year round dense vegetation and higher catchment area, diversity of substrate, river depth may favor the inhabitation of diverse groups of fish.
2.2 Length-weight relationship
The relationship between total length and total weight of V. beso (0.98), G. quadrimaculata (0.92) and G. chebera (0.95) was curvilinear and highly since the r2 varied between 0.92-0.98 (Figure 1) and positively correlated. This indicates that weight of the fishes considered, increased with increase in length. This is in agreement with Fagade and Olaniyan (1972), Fagade (1983), Layèyè (2006) and Ayoade and Ikulala (2007) on different fish species from various water bodies.
|
Figure 1 Length-Weight relationship of V. beso |
The values for the length-weight relationship for G. quadrimaculata and G. chebera were respectively indicated isometric (b=2.614, a=0.02) and (b=2.786, a=0.019) pattern implying that they tend to become thinner with increasing length. But the values obtained for V. beso was (b=2.915, a=0.015) indicated weight increase with length, The b value is nearer to 3 for the fish species selected where the slope ‘b’ may be varied due to stage of sexual maturity, nutritional adequacy of the diet, and toxicology of the environment (Begenal and Tesch, 1978). The results indicated strong positive correlation between the length and weight for V. beso (r2-0.9809), G. chebera (r2-0.9462), and G. quadrimaculata (r2-0.920) at different sites were shown in the (Figure 1; Figure 2; Figure 3).
|
Figure 2 Length-Weight relationship of G. chebera |
|
Figure 3 Length-Weight relationship for G.quadrimaculata |
2.3 Condition factor
In the present study condition factor of V. beso, G. chebera and G. quadrimaculata were calculated both by sites for species and separately for the sexes from all the collection sites. The highest values condition factor was observed for V. beso and G. chebera from the site Deb2 (Table 3). At this station the condition factor of V.beso varied between 0.86 and 2.72 and the mean value was (1.3105 ± 0.23). The condition factor of G. chebera was comparatively high at Deb2, ranged from 0.636 to 2.454 and the mean being 1.3249 ± 0.24.
|
Table 3 Average condition factor values for the fish species in River Debbis |
The mean condition factor of G. quadrimaculata at Deb1 and Deb2 did not vary considerably (1.22 ± 0.21 and 1.20 ± 0.19 respectively), whereas the value at Deb3 was the lowest (1.15 ± 0.18) Condition factor which compares the well-being of a fish is based on the hypothesis that heavier fish of a given length are in better condition (Bagenal and Tesch, 1978). According to Fagade (1979), condition factor decrease with increase in length and also influences the reproductive cycle in fish (Welcome, 1979). The value obtained from the study showed that all species studied were in good condition. The high value of condition factor was observed for V. beso and G. chebera from the site Deb2.
In general the mean K values of female G. chebera were higher than males at all sites (Table 4). However, the mean K values of females G. quadrimaculata was lower than males at Deb1 and Deb3 sites with mean K values 1.23 ± 0.09 and 1.21 ± 0.16 respectively. But, it was higher than males at site two (Deb2) with mean K values 1.20 ± 0.09 (Table 4). Gayanilo and Pauly (1997) reported that certain factors such as, sorting into classes, sex, stages of maturity and state of the stomach often affect the well-being of a fish.
|
Table 4 Condition factor (CF) (Mean ± SD) of selected species |
The condition factor of fish species showed the mean condition factor well above the average of 1.00. The K value recorded in this study agrees with the values reported by Ayoade and Ikulala (2007). This is an indication that the environmental conditions of the river are at optimum level, giving the fish a good condition for growth and development (Wade, 1992). In general, high condition is associated with higher energy (fat) content, increased food base, reproductive potential and more favorable environmental conditions (Paukert and Rogers, 2004). Therefore, the low K value of fishes were probably because of fluctuations in factors such as food quantity and quality, water level, flow rate and water temperature.
2.4 Stomach content analysis
A total of 408 gut samples were examined for food composition. The numbers of specimens examined were V. beso 136, G. chebera 156 and G. quadrimaculata 116. Out of these 38 (9.31%) of the fishes were found with empty stomachs. The food items recorded were diverse groups of phytoplankton, zooplankton, Annelids, detritus and sandy grains (Table 5). The zooplankton such as copepods and cladocera and oligochaete worms were noticed. In addition detritus and sandy grains formed important constituents in the stomach content of the fishes.
|
Table 5 Frequency of occurrence of food items in the gut content of fishes |
All the stomachs of V. beso showed the presence of detritus and sand grains (100%) followed by diatoms with 87.70%. Chlorophyceae (green algae) were found in 31.15% fish stomachs. Zooplankton was collected from 27.86% of the stomachs followed by Cyanophyceae and Oligochaeta in 10.65% of examined stomachs (Table 6). The food items in the stomachs of G. chebera were detritus and sand grains (100%) diatoms (89.58%), Chlorophyceae (25.69%), Zooplankton (18.05%) and Cyanophyceae (13.1%) and Annelids (5.55%) of fishes. Detritus and sandy grains formed the bulk of the ingested item in all the stomachs (100%) of G. quadrimaculata. Diatoms were found in 87.5% of the examined fish, followed by Chlorophyceae 28.8% and Cyanophyceae 16.34%. Zooplankton were observed in (15.38%) of stomachs examined fishes.
|
Table 6 Numerical abundance of food items in the stomach contents |
Numerically, Bacillariophyceae was the most important food item in the stomach of the fishes examined (Table 6) followed by green and blue-green algae. Zooplankton and oligochaete worms were also found occasionally constituted 1.04 and 0.47% of the diet.
Analysis of stomach contents is a method for determining the food and feeding habits of fishes by which we can easily find what the fish take as food. Palmores et al. (1997) stated that stomach content results would help to reduce intra and inter specific competition for ecological niche and in providing straight forward models of stomach content dynamics. In the present study, over 90.69% of the fish specimens showed food in the stomach. The results indicated that river bed is composed of high number of decomposed material and mixed with periphyton composed of large number of diatoms and green algae. The stomach content showed the presence of large amount of sand grains and partially decomposed animal and plant tissues (detritus) suggesting their bottom feeding habit (Ayotunde et al., 2007; Ayoade, 2011). Admassu and Dadebo (1997) and Zerihun et al. (2006) found that L. intermedius in Lake Hawassa feeds on phytoplankton, insects, detritus, macrophytes, gastropods and fish. According to Assaminew (2005), the diet of L. intermedius was composed of macrophytes, detritus, insects, nematodes, fish, fish eggs and fish scales in Lake Koka. In the present study also the cyprinid fish V. beso feeds mainly on diatoms and green algae along with detritus of diverse origin in the Debbis River.
2.5 Fishery activities
Majority of the fishers in the study area (61.2%) have involved in fishing for 6-15 and there are seasonal fishermen who fish from the river only for consumption. Fishing in the study area is mainly artisanal and sold at the local market. The commercially important species are L. forskalii, V. beso and L. intermedius. The fisher folk use locally available gears such as single hook and line and cast net (8-10 cm mesh size). Besides, Birbira (Milletia ferruginea) seed powder is also used to anesthetize and collect the fishes.
3 Materials and Methods
The study area is located between 09°00’149’N latitude and 37°49’783’E longitude and altitude between 1886-1919 m.a.s.l in the West Shoa Zone in Ambo and Tokke kutaye districts, Ethiopia. The river bank is covered with dense vegetation and rocky hills and agricultural lands. Livestock rearing is an important activity besides crop production by the people. Fishing is practiced along Debbis River by fishermen to meet subsistence needs as an additional source of income. Fishing is carried out during December to April when the water level becomes less. The three representative sampling sites were selected on Debbis River and designated as (Deb1, Deb2 and Deb3) based on nature of bottom and the surroundings (Figure 4). Fish samples were collected from each site (pools/riffles) during the dry season from January to March, 2017 by Electrofishing. Immediately after capture, total length and total weight of all fishes were measured. Identification of the fish species was carried out following Fish Base (2006) software.
|
Figure 4 Map of the study area showing the sampling sites along the Debbis River |
Diversity Index was used to evaluate diversity of fish species. The Shannon index (H’) is a measure of species weighted by the relative abundance of the species of fish (Begon et al., 1990).
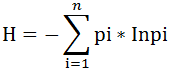
Where the proportion (n/N) of individuals of one particular species; (n) = the total number of a particular species; N = the total number of all the species; ln is the natural log; Σ is the sum of the calculations.
The relationship between total length and total weight of the most dominant species was calculated using power functions as in Bagenal and Tesch (1978) as follows:
.png)
Where TW = Total weight (gm.); TL = Total length (cm); a = Intercept of the regression line; b = Slope of the regression line.
The well-being of the most dominant species was determined using Condition Factor (K) by the following equation suggested by Bagenal and Tesh (1978).
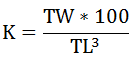
Where TW = Total weight; TL = Total length; K = Constant value.
The fish stomach contents were analyzed following numerical and frequency of occurrence methods (Hynes, 1950; Hyslope, 1980; Ugwumba and Ugwumba, 2007). Purposive sampling technique was employed to select the districts based on fishing activities along the river. Totally 44 fishers (seasonal fishermen and farmers) were interviewed to collect data on the fish catch and marketing.
Authors’ contributions
Kebede Tirfessa made the proposal and conducted the study. Dr. L. Prabhadevi being the mainadvisor corrected the manuscript. Dr. Zenebe Tedesse was the co-advisor, assisted in the collection and identification of fishes and evaluated the manuscript for presentation.
Acknowledgements
The authors are grateful to the Director, Fishery and Aquatic life research center, Sebeta, Ethiopia for providing the electro fishing gadget for the collection of fishes.
Abegaz H., Tesfaye G., and Cheffo A., 2010, Fishery Development Program: Riverine Fishery Assessment in Gambella Peoples’ Regional State, Report for Agricultural Extension Directorate, Ethiopia. Agricultural Extension Directorate Ministry of Agriculture pp. 68
Ameha A., and Assefa A., 2002, The fate of the barbs of Gumara River, Ethiopia. SINET: Ethiopian Journal of Science, 25(1): 1-18
https://doi.org/10.4314/sinet.v25i1.18069
Assaminew K, 2005, Distribution, abundance and feeding biology of fish species in Koka reservoir and the associated Awash River floodplain, Ethiopia
Ayoade A.A., and Ikulala A.O.O., 2007, Length-weight relationship, condition factor and stomach content of Hemichromis bimaculatus, Sarotherodan melanotheron and Chromidotilapia guentheri (Perciformes: Cichlidae) in Eleiyele Lake, South-western Nigeria, International Journal of Tropical Biology, 55(3-4): 969-977
http://www.scielo.sa.cr/pdf/rbt/v55n3-4/art20v55n3-4.pdf
Ayotunde E.O., Stephen N.O., and Okey I.B., 2007, Parasitological examinations and food composition in the gut of feral African Carp, Labeo coubie in the Cross River, Southeastern Nigeria, African Journal of Biotechnology, 6(5): 625-630
http://www.academicjournals.org/journal/AJB/article-full-text-pdf/DF8F4756614
Ayoade A.A., 2011, Length-weight relationship and diet of African Carp, Labeo ogunensis (Boulenger, 1910) in Asejire Lake, South western Nigeria, Journal of Fisheries and Aquatic Science, 6(4): 472-478
https://doi.org/10.3923/jfas.2011.472.478
BagenalT.B. and Tesch, F.W. ,1978, Age and growth. In: Bagenal, T.B. (ed). Methods for Assessment of fish production in fresh waters, 101-136, Black well, Oxford, New York
Berie Z., 2007, Diversity, relative abundance and biology of fishes in beles and gelgel beles rivers, Abay basin Ethiopia. M.Sc. Thesis, Addis Ababa University, Ethiopia.and challenges emerging from the rise of aquaculture. Journal of Fish Biology (83):1067-1084
https://doi.org/10.1111/jfb.12187
De Graaf M., 2003, Lake Tana's piscivorous Barbus (Cyprinidae, Ethiopia) ecology-evolution-exploration, doctoral thesis experimental zoology group, Wageningen University, Wageningen, The Netherlands
http://library.wur.nl/WebQuery/wurpubs/fulltext/121409
Edds D.R., 1993, Fish assemblage structure and environmental correlates in Nepal's Gandaki River, Copeia, 1: 48-60
https://doi.org/10.2307/1446294
Fagade S.O., 1979, Observation on the species of two species of Tilapia from the Lagos, Lagoon, Nigeria. Bulletin de l’l FANT, 41 Ser A No 3
Fagade S.O., 1983, The biology of Chromidotilapia guentheri from a small lake, Archiv fur Hydrobiology, 97: 60-72
http://agris.fao.org/agris-search/search.do?recordID=US201302128218
Fagade S.O., and Olaniyan C.I.O., 1972, The biology of the west African shad Ethmalosa fimbriata (Bodwich) in the Lagos Lagoon, Nigeria, Journal of Fish Biology, 4(4): 519-533
https://doi.org/10.1111/j.1095-8649.1972.tb05699.x
Fish Data Base Program, Froese, R, Pauly D. Concepts, design and data sources 2012, Available on
http://www.fishbase.org/search.php
Food and Agriculture Organization (FAO), 2004, The state of world fisheries and aquaculture, part 4, Outlook, editorial productions and Design group, Food and Agriculture Organization of United Nations, Rome, Italy
Gayanilo F.C., and Pauly D., 1997, FAO ICLARM stock assessment tools (FISAT): References Manual, FAO Comput. Inf. Ser. Fish. (8): pp. 262
Getahun A., 2005a, Freshwater Eco-regions of Ethiopia. In: Theime et al. (eds), Freshwater eco-region of Africa. A conservation assessment/Island press, Washington, D.C., U.S.A
Getahun A., 2005b, An over view of the diversity and conservation status of the Ethiopian fresh water fish fauna. In: proceeding of the Pan-African Fish and Fisheries Society, Cotonou, Benin, Nov. 2003
Golubtsov A. S., and Mina, M. V., 2003, Fish species diversity in the main drainage systems of Ethiopia: current state of knowledge and research perspectives, Ethiop. J. Natu. Reso, 5(2): 281-318
Habteselassie R., 2012, Fishes of Ethiopia, Annotated Checklist with Pictorial Identification Guide, Ethiopian Fisheries and Aquatic Science Association, Addis Ababa, Ethiopia. pp. 250
Hynes H.B.N., 1950, The food of the freshwater sticklebacks (Gastrosteus aculeatus and Pygosteus pungitius) with a review of methods used in studies of the food of fishes, The Journal of Animal Ecology, 19: 36-58
Hyslop E.J., 1980, Stomach content analysis-a review of methods and their application, Journal of Fish Biology 17(4): 411-429
https://doi.org/10.1111/j.1095-8649.1980.tb02775.x
Lalèyè P.A., 2006, Length-weight and length-length relationships of fishes from the Ouèmè River in Benin (West Africa), Journal of Applied Ichthyology, 22(4): 330-333
https://doi.org/10.1111/j.1439-0426.2006.00752.x
Meador M.R., Cuffney T.F., and Gurtz M.E., 1993, Methods for sampling fish communities as part of the National Water-Quality Assessment program: U.S. Geological Survey Open-File Report, No.93-104, pp.40
http://pubs.er.usgs.gov/publication/ofr93408
MOARD, 2009, Ministry of Agriculture and Rural Development DAs & FTC, Data at National Level, Addis Ababa, Ethiopia: MOARD
Nikolsky G.V., 1963, The Ecology of Fishes, Academic Press, London and New York, pp. 352
Oumer M., Mingist M., Dejen E., 2011, Diversity and relative abundance of fishes in the head of Blue Nile River, Ethiopia, Ethiopian Journal of Biological Sciences, 10(2): 207-212
Okoh R.N., Ugwumba C.O.A., and Elue H.O., 2008, Gender roles in Food stuff marketing in DeltaNorth Agricultural Zone: The case of Rice. In: Ume et al. (eds), 22nd Annual National Conference Proceedings, pp. 114-123
Paukert C., and Rogers R.S., 2004, Factors affecting condition of flannel mouth suckers in the Colorado River, Grand Canyon, Arizona, North American Journal of Fisheries Management, 24(2): 648-653
https://doi.org/10.1577/M03-087.1
Tedla S., 1973, Freshwater Fishes of Ethiopia; Haile Selassie I University, Dept. of Biology, Addis Ababa, Ethiopia, pp. 107
Tesfaye G., and Wolff M., 2014, The state of inland fisheries in Ethiopia: A synopsis with updated estimates of potential yield, Ecohydrology & Hydrobiology, 14(3): 200-219
https://doi.org/10.1016/j.ecohyd.2014.05.001
Tigabu Y., and Abegaz H., 2007, Reconnaissance survey on the river fisheries of Benishangul-gumuz regional state, Proceedings of the 15th Annual conference of the Ethiopian Society of Animal Production (ESAP) held in Addis Ababa, Ethiopia; October 4-6, ESAP, Addis Ababa, 129-142
http://www.eap.gov.et/sites/default/files/ESAP_proceeding-15th-tech..pdf#page=137
Toham A.K., and Teugels G., 1998, Diversity pattern of fish assemblages in the lower Ntem River basin (Cameroon), with notes on potential effects of deforestation, Arch. Hydrobiology, 141(4): 421-446
https://doi.org/10.1127/archiv-hydrobiol/141/1998/421
Ugwumba A.A., and Ugwumba O.A., 2007, Food and feeding Ecology of Fishes in Nigeria, Crystal publishers, Lagos, Nigeria, 5-6
http://afrilib.odinafrica.org/handle/0/13775
Welcome R.L., 1979, Fisheries ecology of Flood Plain Rivers, Longman G, London
. PDF(522KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Kebede Tirfessa Urga
. L. Prabhadevi
. Zenebe Tedesse
Related articles
. Diversity
. Debbis River
. Condition factor
. Frequency of occurrence
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)


