Research Report
Effect of Stocking Density on the Survival and Growth of Hoplobatrachus occipitalis (Gunther, 1858) (Amphibia: Dicroglossidae) of Tadpoles Reared in Ponds from Benin 
2 Laboratoire d’Hydrobiologie, UFR Biosciences, Universite Felix Houphouet-Boigny, 22BP: 582 Abidjan 22, Cote d’Ivoire
 Author
Author  Correspondence author
Correspondence author
International Journal of Aquaculture, 2018, Vol. 8, No. 18 doi: 10.5376/ija.2018.08.0018
Received: 14 May, 2018 Accepted: 28 Jun., 2018 Published: 24 Aug., 2018
Godome T., Tossavi E., Djissou A., Zounon Y., Ouattara N.I., and Fiogbe E.D., 2018, Effect of stocking density on the survival and growth of Hoplobatrachus occipitalis (Günther, 1858) (Amphibia: Dicroglossidae) of tadpoles reared in Ponds from Benin, International Journal of Aquaculture, 8(18): 137-144 (doi: 10.5376/ija.2018.08.0018)
Density is one of the important factors to be considered in frogs rearing. The current study aims to evaluate the effect of this parameter on the survival and growth of Hoplobatrachus occipitalis tadpoles in circular plastic ponds (Diameter: 24 cm; volume: 60 L) for 24 days. Four stocking densities (5, 10, 15 and 20 tadpoles/L), respectively represented by D1, D2, D3, and D4 were tested on tadpoles (initial mean weight 0.283 ± 0.031 g) in triplicate. Tadpoles were daily fed at 6% of biomass every hour from 8 a.m to 6 p.m on Coppens fish feed. At the end of experiment, parameters such as final biomass (9.386 ± 0.240 to 23.750 ± 0.601 g), specific growth rate (1.960 ± 0.326 to 4.220 ± 0.255%/d-1), daily weight gain (0.022 ± 0.021 to 0.735 ± 0.026 g/d), survival rate (30.388 ± 1.734 to 86.333 ± 3.333%) and feed conversion rate (1.436 ± 0.023 to 2.185 ± 0.218) showed the lowest values in the highest stocking densities. The final biomass, specific growth rate, daily weight gain and survival rate recorded in D1 tadpoles were significantly higher (p˂0.05) compared to the other lots. Thus the optimal stocking density is 5 tadpoles/L to ensure a good growth of H. occipitalis tadpoles in circular ponds.
Background
The increase in world-wide population by the later years requires an increase in natural resources such as water, energy and food (Beddington, 2011). For that purpose, new rearing approaches that enable reduction of environmental pollution and maximizing the synthesis of high quality biological protein were envisaged (Munguia-Fragozo et al., 2015). Nevertheless, the rearing of Hoplobatrachus occipitalis is a trust able solution to satisfy this demand. However, due to its complex life cycle, many rearing methods must be applied in each stage of its life. In aquaculture, two factors determine the available biomass quantity: individual growth and the number of individuals (Flores-Nava and Vera-Muñoz, 1999). These two factors are often inversely proportional as growth is generally influenced by rearing density (Flores-Nava and Vera-Muñoz, 1999). Though there is an incomplete understanding of why density reduces growth and provokes metamorphosis of small height tadpoles, the effect on growth and development at different densities is actual and measurable (Wilbur and Collins, 1973).
In frog rearing, growth and metamorphosis time of tadpoles are affected by the stocking density (Wilbur, 1977; Collins, 1979; Hota and Dash, 1981; Fontanello et al., 1988). Reservoirs’ volume and content (individual number) is one of the noteworthy questions to be studied. Negative effects of growth of density on the frogs growth rate and development were studied by many authors (Licht, 1967; Wilbur, 1977b; Smith-Gill and Berven, 1979; Travis, 1981; Semlitsch and Caldwell, 1982).
Besides that, several researches focused on the food diet (Alfredo, 1996; Mady-Goma, 2012), biology (Wells, 1977; Barbault, 1984; Heyer et al., 1994; Rödel, 2000; Morin, 2008; Tohé, 2009), ecology (Rödel, 2000; Wildlife et al., 2007; Tohé, 2009) of H. occipitalis but dids relative to stocking density of this species remain narrow. The aim of this study is to evaluate the effect of stocking density on the survival and growth of H. occipitalis (Günther, 1858) tadpoles in controlled medium from Benin.
1 Material and Methods
1.1 Experimental process
Tadpoles of H. occipitalis used in the current experiment were obtained by semi-artificial reproduction on the site of the Research Laboratory on Wetlands (LRZH) of the University of Abomey-Calavi. Mean value of the water temperature, pH and dissolved oxygen of the semi-artificial reproduction medium were respectively 28.33 ± 0.09°C, 7.01 ± 0.01 and 3.98 ± 0.02 mg/L. A week after hatching, tadpoles harvested with dip nets were transferred in experimental circular plastic ponds. They were fed on live food (zooplankton and nauplii of Artemia salina). Live food was progressively substituted by commercial feed (Coppens) according to Table 1.
|
Table 1 Planning of Hoplobatrachus occipitalis tadpoles feeding in closed circuit in the laboratory Note: Artificial feed = Coppens 0.5-0.8 mm, 56% protein and 15% lipid |
The experiment was led for 24 days in 12 circular plastic ponds (Diameter: 24 cm; volume: 60 L) containing 6 L water each. Tadpoles (initial mean weight 0.284 ± 0.033 g) were distributed in four different densities: 5 tadpoles/L (D1), 10 tadpoles/L (D2), 15 tadpoles/L (D3) and 20 tadpoles/L (D4). Water renewal was continuous. Densities were chosen based on the preliminary experiments on the stocking density of other frog species (Murray, 1990; Martìnez et al., 1996; Flores-Nava and Vera-Muñoz, 1999). Tadpoles were daily fed manually every 2 hours from 8 a.m to 6 p.m on Coppens fish feed (0.5-0.8 mm diameter, 56% protein and 15% lipid) at 6% of body weight.
During the experiment, remained feed and feces were siphoned twice a day before feeding and renewal, at 7:30 a.m and 6:30 p.m. After siphoning, water volume in each pond was adjusted and dead tadpoles were counted and weighted.
Water quality monitoring was carried out by the measurement of physico-chemical parameters such as temperature, dissolved oxygen and pH. To measure these parameters, a portable multi meter (Calypso ORCHIDIS SN-ODEOA 2138) was used (7:30 a.m and 5:30 p.m). Nitrites (N-NO2, according to diazotization method), nitrates (N-NO3, according to cadmium reduction method) and ammoniac (N-NHde, according to salicylate method) were measured by using a spectrophotometer (HACH). Control fishing was carried out every 3 days following by ponds emptying and cleaning. Number and biomass of tadpoles were measured by pond. Feed ration was adjusted consequently. After 24 days rearing, all tadpoles were collected from ponds and weighted. Zootechnical performances such as Specific Growth Rate (SGR), Survival Rate (SR), Daily Weight Gain (DWG) and Feed Conversion Rate (FCR) were calculated according to the following formula:
.png)
.png)
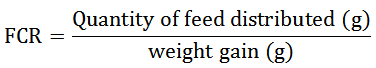
.png)
1.2 Statistical analysis
Data collected were encoded in Microsoft Excel 2013. They served to calculate zootechnical parameters. For each parameter, mean and range were calculated. Mean data for each replication were considered as an observation.
The statistical software R (version 3.4.2) was used for analyses with 5% probability significance threshold. A one way analysis of variance (ANOVA 1) was carried out in order to compare zootechnical parameters in the different treatments. The Student-Newman-Keuls test (SNK test) served to carry out pair comparisons of the different treatments when significant differences were observed among treatments to determine the optimal density, growth and survival rate of H. occipitalis tadpoles.
2 Results
Table 2 shows the water physico-chemical parameters such as temperature, pH, dissolved oxygen, conductivity, nitrites (N-NO2), nitrates (N-NO3) and ammoniac (N-NH3). During the experiment period, there was no significant difference (p>0.05) among temperature, pH and conductivity. The highest values of these latter were respectively 30.70°C (D3), 6.4 (D1) and 688.4 µs/cm (D4) while the lowest 25.22°C (D2), 5.2 (D4) and 410.6 µs/cm (D2). Concerning nitrites and nitrates, significant differences (p˂0.05) were observed among D1 and D3, D4. In contrary, there was no significant difference (p>0.05) among D2, D3 and D4. Concerning total Ammoniac, significant differences were observed (p˂0.05) among D4 and D1, D2, D3.
|
Table 2 Mean ± range of temperature, pH, dissolved oxygen (DO), conductivity, nitrates, nitritres and ammoniac in ponds water during the rearing period with the different stocking densities Note: abc mean values on the same line and not affected by the same letter are significantly different (p<0.05) |
Growth performances, stocking densities and survival rate of H. occipitalis tadpoles are mentioned in Table 3. Final biomass, specific growth rate, food conversion rate and daily weight gain were considerably influenced by the stocking density (Table 3). The increase of individual number in ponds led to a decrease of tadpoles’ growth performance. The maximal mean value of final biomass was observed in density D1 (5 tadpoles/L) either 23.750 ± 0.871 g. The lowest mean value was recorded in D4 (20 tadpoles/L).
|
Table 3 Mean ± range of Initial Biomass (IB), Final Biomass (FB), Specific Growth Rate (SGR), Food Conversion Rate (FCR), Survival Rate (SR) and Daily Weight Gain (DWG) of Hoplobatrachus occipitalis tadpoles reared at different stocking density Note: abc mean values on the same line and not affected by the same letter are significantly different (p<0.05) |
Specific Growth Rate (SGR), Survival Rate and Food Conversion Rate (FCR) were significantly affected (p˂0.05) by stocking densities of H. occipitalis tadpoles. Tadpoles reared in high density (10 to 20 larvae/L) showed a low specific growth rate (1.960 ± 0.326 to 3.683 ± 0.561%/d) (Figure 1). In return, those reared at 5 tadpoles/L showed a high specific growth rate (4.220 ± 0.255%/d) (Table 3). Survival rate of H. occipitalis tadpoles varied between 30.388 ± 1.734% (D4) and 86.333 ± 3.333% (D1). Moreover, tadpoles were significantly (p˂0.05) affected by the different stocking densities. Concerning the food conversion rate, there was no significant difference between D3 and D4. Nevertheless, there was a significant difference (p˂0.05) among mean values of D1 and D2, D3, D4 (Figure 2).
|
Figure 1 Specific growth rate of Hoplobatrachus occipitalis tadpoles reared at four (04) stocking densities in ponds for 24 days |
|
Figure 2 Relationship between stocking density and survival rate of H. occipitalis tadpoles reared in ponds for 24 days |
3 Discussion
During the experiment, mean value of temperature (26.57°C) was situated in the favorable limit recommended by Petersen and Gleeson (2011) for tadpoles rearing (15 to 35°C). Mean values of pH and dissolved oxygen recorded during this experiment were similar to those recorded in prior studies (Flores-Nava and Vera-Muñoz, 1999) in Rana catesbeiana Shaw (1802) tadpoles. Besides, lowest values of pH and dissolved oxygen were obtained in ponds with high tadpoles stocking densities (D3 and D4). These low dissolved oxygen rates may result from high oxygen consumption by tadpoles (Browne et al., 2003). Mean values of total ammoniac and nitrites were similar to those recorded by Flores-Nava and Vera-Muñoz (1999) and Munguia-Fragozo et al. (2015). Concerning nitrates, mean values were similar to those obtained by Munguia-Fragozo et al. (2015). These results could be explained not only by remain feed and tadpoles feces that degrade in the rearing media but also the ammoniac produced these remain feed and feces. Ammoniac is a highly toxic element that may be the reason of mortality observed in high densities media. It’s important to notice that nitrates (NO3-) are less toxic than ammoniac and nitrites (Aquarioplus, 2018).
In experimental conditions, the current study shows that H. occipitalis tadpoles develop as well as metamorphosis when they are reared at density of five (05) individuals per liter of water. This density is nearby the normal recommended for R. catesbeiana tadpoles in commercial farms (Lopes-Lima and Agostinho, 1992; Flores-Nava, 1997). Besides, the highest final mean weight (0.735±0.026 g) in this experiment was recorded in D1 (05 tadpoles/L) (Figure 3). These results are closed to those obtained (0.449-1.383 g) by Martìnez et al. (1996) during their study on growth and metamorphosis of Rana perezi Seoane (1885) tadpoles. These results are also similar to those obtained (0.403-0.716 g) by Murray (1990) in Rana sylvatica tadpoles. Although, these latter are lower than the results of Browne et al. (2003) and Munguia-Fragozo et al. (2015) during their studies on the effect of high density on growth, development and survival of Litoria aurea tadpoles and the effect of density on growth and metabolism of R. catesbeiana tadpoles. This may due not only to the difference of species but the difference of rearing techniques. Indeed, it was demonstrated that space availability can influence tadpoles’ development (Flores-Nava and Vera-Muñoz, 1999). According to Wilbur and Collins (1973), tadpoles reared at low stocking densities are able to pass minimal body height to initiate metamorphosis process and maximize their height after. Nevertheless, it can be difficult to compare precisely these studies because Browne et al. (2003) studied L. aurea in Australia and Munguia-Fragozo et al. (2015) focused on R. catesbeiana in Mexico. Factors to be considered are for instance temperature variations, differences in experimental designs and water quality management.
|
Figure 3 Biomass progression of Hoplobatrachus occipitalis tadpoles reared in ponds at four (04) stocking densities for 24 days |
Specific growth rate recorded during the current experiment ranged between 1.960 ± 0.326 and 4.220 ± 0.255%/d (Figure 1). These results are closed to those of many authors (Martìnez et al., 1996; Flores-Nava and Vera-Muñoz, 1999) Rana perezi and Rana catesbeiana tadpoles. Besides, many studies indicate specific growth rate of tadpoles are negatively tied to the intensity of inter specific competition observed in high density media (Wilbur and Collins, 1973; Crump, 1981; Semlitsch and Caldwell, 1982; Berven and Chadra, 1988; Tejedo and Reques, 1992). Our results show specific growth rate of H. occipitalis tadpoles is inversely proportional to density. The best results were obtained in ponds with low stocking density (5 tadpoles/L). That confirms results of Browne et al. (2003) who showed high stocking densities of tadpoles provokes stress conditions which affect their survival and growth (height and metamorphosis time). According to Semlitsch and Caldwell (1982), specific growth rate decreases while density increases. Besides, water renewal allows a good growth of tadpoles. For example, prior studies revealed that in R. catesbiana water renewal drops temperature thus favor good growth performance (Flores-Nava et al., 1994).
During the experiment, food conversion rate of H. occipitalis tadpoles varied from 1.436 ± 0.023 and 2.185 ± 0.218. The best rate was recorded in density of 5 tadpoles/L. These results are different from those obtained by Flores-Nava and Vera-Muñoz (1999) Rana catesbeiana tadpoles (0.5 tadpoles/L). This could be justified by social interactions existing between feed and space. Indeed, in several reared species, growth is inversely proportional to density, what is principally attributed to social interactions (Siddiqui et al., 1989, Irwin et al., 1999). We also supposed in our study that equal feed particles were consumed by each tadpole at each feeding time. Moreover, they were some competitive interactions in ponds limiting tadpoles’ equal access to feed. It’s important to notice that high stocking density increases constraints (aggressive behavior, dominance), that provokes high energy needs leading to the reduction of food conversion rate (Suziki et al., 2001; Begout-Anras and Lagardere, 2004).
The effects of density on survival were demonstrated by several authors (Wilbur, 1977; Hota and Dash, 1981). This latter depends on the species (Pangni et al., 2008). In the current study, survival rate of H. occipitalis tadpoles (86.333 ± 3.333 to 30.388 ± 1.734%) decreased while stocking density increased (Figure 2). Similar results were recorded in R. catesbeiana tadpoles (Munguia-Fragozo et al., 2015), P. saharicus (Meher et al., 2014), L. aurea (Browne et al., 2003). Although, our results are lower than those recorded in R. catesbeiana (Flores-Nava and Vera-Muñoz, 1999) and those of Martìnez et al. (1996) in R. perezi. These results may due to experimental conditions of rearing media and climate. The high survival recorded in D1 could be attributed to favorable environmental conditions during the experiment period. This is in accordance with results of El-Sherif and El-Feky (2009) who indicated highest survival rates could be tied to favorable ecological conditions.
4 Conclusion
At the end of the current study, it results stocking density affects specific growth rate, survival rate and feed conversion rate of H. occipitalis tadpoles in the different ponds. Those maintained at high densities (10 to 20 tadpoles/L) had low growth that indicates disfavorable rearing conditions of tadpoles. The density of 5 tadpoles/L is recommended so that to reduce stress and improve maximal growth performances of H. occipitalis tadpoles.
Authors’ contributions
All authors, TG, ET, AD, YZ, NIO, and EDF, have made adequate effort on all parts of the work necessary for the development of this manuscript according to his expertise. All authors read and approved the final manuscript.
Acknowledgements
The authors thank World Bank through African Center of Excellence on Climate, Biodiversity and Sustainable Agriculture (CEA-CCBAD) for financial support for the scholarship granted to Théophile GODOME. We thank, Azon M.T. Césaire, Zannou G. James and Alapini Landry for her contribution to this work. The authors thank also the reviewers for their contribution in improve the scientist quality of this manuscript.
Alfredo S., 1996, Amphibians of Northwest Africa, pp.45
Aquarioplus, 2018, Cycle de l'azote, pp.2
Barbault R., 1984, Stratégie de reproduction et démographie de quelques amphibiens anoures tropicaux, Oikos, 43: 77-87
https://doi.org/10.2307/3544248
Beddington J., 2011, The future of food and farming, International Journal Agricultural Management, 1: 2-6
Bégout A.M.L., and Lagardère J.P., 2004, Domestication et comportement chez les poissons téléostéens, INRA Production Animales, 17(3): 211-215
Berven K.A., and Chadra B.G., 1988, The relationship among egg size, density and food level on larval development in the wood frog (Rana sylvatica), Oecologia, 75: 67-72
https://doi.org/10.1007/BF00378815
Browne R.K., Pomering M., and Hamer A.J., 2003, High density effects on the growth, development and survival of Litoria aurea tadpoles, Aquaculture, 215: 109-121
https://doi.org/10.1016/S0044-8486(02)00205-3
Collins J.P., 1979, Intrapopulation variation in body size at metamorphosis and timing of metamorphosis in the bullfrog Rana catesbeiana, Ecology, 60: 738-749
https://doi.org/10.2307/1936611
Crump M.L., 1981, Energy accumulation and amphibian metamorphosis, Oecologia, 49: 167-169
https://doi.org/10.1007/BF00349184
El-Sherif M.S., and El-Feky A.M.I., 2009, Performance of Nile tilapia (Oreochromis niloticus) fingerlings, I. Effect of pH, International Journal of Agriculture & Biology, 11: 297-300
Flores-Nava A., and Vera-Muñoz P., 1999, Growth, metamorphosis and feeding behaviour of Rana catesbeiana Shaw 1802 tadpoles at different rearing densities, Aquaculture Research, 30, 341-347
Fontanello D., Arruda H., Mandelli J., Campos B., Penteado L.A., and Prandi D., 1988, Developmental control of bullfrog tadpoles (Rana catesbeiana Shaw, 1802) for commercial cultivation: population density, protein quality in the ration and tank localization, Boletim do Instituto de Pesca, 15: 19-24
Heyer W.R., Donnelly M.A., Mcdiarmid R.W., Hayek L.A.C., and Froster M.S., 1994, Measuring and monitoring biological diversity, Standard methods for amphibians, Washington & London, Smithsonian Institution Press, pp.364
Hota A.K., and Dash M.C., 1981, Growth and metamorphosis of Rana tigrina larvae: effects of food level and larval density, Oikos, 37: 349-352
https://doi.org/10.2307/3544127
Irwin S, Halloran J.O., and Fitz-Gerald R.O., 1999, Stocking density, growth and growth variation in juvenile turbot (Scophthalmus maximus), Aquaculture, 178: 77-88
https://doi.org/10.1016/S0044-8486(99)00122-2
Licht L.E., 1967, Growth inhibition in crowded tadpoles: intraspecific and interspecific effects, Ecology, 48: 736-745
https://doi.org/10.2307/1933731
Lopes-Lima S., and Agostinho C.A., 1992, A tecnología de criaçao de ras, Universidade Federal de Viçosa, Imprenta Universitaria, Viçosa, Minas Gerais, Brazil, pp.168
Mady-Goma D.I., Kimpoudi C., Mikia M., Tsoumou A., Vouidibio J., and Pandare D., 2012, Study of an Edible frog of brazzaville: Hoplobatrachus occipitalis: Ranidae (Günther, 1858), International Research Journal of Biological Sciences, 1(6): 10-17
Martinez I.P., Rafael À., and Herráez P.M., 1996, Growth and metamorphosis of Rana perezi larvae in culture: effects of larval density, Aquaculture, 142: 163-170
https://doi.org/10.1016/0044-8486(96)01257-4
Meher B., André N., Mouna F.B., Hechmi M., and Lotfi A., 2014, Effects of temperature, density and food quality on larval growth and metamorphosis in the north African green frog Pelophylax saharicus, Journal of Thermal Biology
https://doi.org/10.1016/j.jtherbio.2014.08.006
Morin R., 2008, Élevage de la grenouille, Document d'information DADD-10, Ministère de l'Agriculture, des Pêcheries et de l'Alimentation, pp.9
Munguia-Fragozo P.V., Alatorre-Jacome O., Aguirre-Becerra H., García-Trejo J.F., Soto-Zarazúa G.M., and Rico-García E., 2015, Growth and metabolic effects of stocking density in bullfrog tadpoles (Rana catesbeiana) under greenhouse conditions, International Journal of Agriculture and Biology., 17(4): 711-718
https://doi.org/10.17957/IJAB/14.0002
Murray D.L., 1990, The effects of food and density on growth and metamorphosis in larval wood frogs (Rana sylvatica) from central Labrador, Canadian Journal Zoology, 68: 1221-1226
https://doi.org/10.1139/z90-182
Pangni K., Atsé B.C., and Kouassi N.J., 2008, Effect of stocking density on growth and survival of the African catfish Chrisichthys nigrodigitatus, Claroteidae (Lacépède 1803) larvae in circular tanks, Livestock Research for Rural Development, 20(7)
Petersen A.M., and Gleeson T.T., 2011, Acclimation temperature affects the metabolic response of amphibians skeletal muscle to insulin, Comparative Biochemistry and Phisiology, 160(3): 72-80
https://doi.org/10.1016/j.cbpa.2011.05.005
Rödel M.O., 2000, Herpetofauna of West Africa, vol. i: amphibians of the West African savanna, Edition chimaira, frankfurt/m, pp.335
Semlitsch R.D., and Caldwell J.P., 1982, Effects of density on growth, metamorphosis, and survivorship in tadpoles of Scaphiopus holbrooki, Ecology, 63: 905-911
https://doi.org/10.2307/1937230
Siddiqui A.Q., Howlader M.S., and Adam A.B., 1989, Culture of Nile tilapia, Oreochromis niloticus (L.), at three stocking densities in outdoor concrete tanks using drainage water, Aquacult. Fish. Manag., 20: 49-58
https://doi.org/10.1111/j.1365-2109.1989.tb00440.x
Smith-Gill S.J., and Berven K.A., 1979, Predicting amphibian metamorphosis, American Naturalist., 113: 563-585
https://doi.org/10.1086/283413
Suziki N., Kondo M., Gunes E., Ozongun M., and Ohno A., 2001, Age and growth of turbot Psetta maxima in the Black Sea, Turk, Journal of Fisheries and Aquatic Science, 1(1): 43-53
Tejedo M., and Reques R., 1992, Effects of egg size and density on metamorphic traits in tadpoles of the natterjack toad (Bufo catamita), Journal of Herpetology, 26: 146-152
https://doi.org/10.2307/1564855
Tohé B., 2009, Reproduction et régime alimentaire de trois espèces d'anoures des habitats dégrades du Parc National du Banco (Côte d'Ivoire): Ptychadena mascareniensis, P. pumilio et Hoplobatrachus occipitalis, -PhD thesis, Université d'Abobo-Adjamé, Abidjan, Côte d'Ivoire, pp.132
Travis L., 1981, Control of larval growth variation in a population of Pseudacris triseriata (Anura: Hylidae), Evolution, 35: 423-432
https://doi.org/10.2307/2408191
Wells K.D., 1977, The social behaviour of anuran amphibians, Animal Behaviour, 25: 666-693
https://doi.org/10.1016/0003-3472(77)90118-X
Whitfield S.M., Bell K.E., Philippi T., Sasa M., Bola-os F., Chaves G., Savage J.M., and Donnelly M.A., 2007, Amphibian and reptiles declines over 35 years at La Selva, Costa Rica, Proceedings of the National Academy of Sciences (PNAS), 104(20): 8352-8356
https://doi.org/10.1073/pnas.0611256104
Wilbur H.M., 1977, Interactions of food level and population density in Rana sylvatica, Ecology, 58: 206-209
https://doi.org/10.2307/1935124
Wilbur H.M., 1977, Density-dependant aspects of growth and metamorphosis in Bufo americanus, Ecology, 58: 196-200
https://doi.org/10.2307/1935122
Wilbur H.M., and Collins J.P., 1973, Ecological aspects of amphibian metamorphosis, Science, 182: 1305-1314
. PDF(376KB)
. FPDF
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Theophile Godome
. Ephrem Tossavi
. Arnauld Djissou
. Yaovi Zounon
. Nahoua Issa Ouattara
. Emile Didier Fiogbe
Related articles
. Hoplobatrachus occipitalis
. Intensive rearing
. Tadpoles
. Stocking density
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)


