Research Article
Ovarian Development and Sex Steriod Hormones in the Keeled Fish Liza carinata (Valenciennes, 1836) From Suez Bay, Egypt 
2 Zoology Department, Faculty of Science, Port Said University, Port Said, Egypt
3 Department of Reproductive Physiology, Central Laboratory for Aquaculture Research, Agriculture Research Center, Egypt
4 Marine Science Department, Faculty of Science, Port Said University, Port Said, Egypt
 Author
Author  Correspondence author
Correspondence author
International Journal of Aquaculture, 2016, Vol. 6, No. 6 doi: 10.5376/ija.2016.06.0006
Received: 22 Mar., 2016 Accepted: 20 May, 2016 Published: 15 Nov., 2016
Hefny A.S.E.D., Elhalfawy M.M., Abass O.A., Wafeek M., and El-Regal M.A.A., 2016, Ovarian development and sex steriod hormones in the keeled fish liza carinata (valenciennes, 1836) from Suez Bay, Egypt, International Journal of Aquaculture, 6(6): 1-10 (doi: 10.5376/ija.2016.06.0006)
This work aims to investigate the microscopic structures of ovaries of Liza carinata collected from Suez Bay during annual cycle combined with the relation between GSI (gonadosomatic index) and sex steroid hormones concentration for female during the study period. The oocyte maturation showed the following phases; first growth phase which includes chromatin-nucleolus stage, early and late perinucleolus stages. Second growth phase includes vacuolization stage (yolk vesicle stage), yolk stages (primary, secondary and tertiary), and ripe stage. The maturity stages of ovary recorded and classified into seven stages (Immature, Immature & Recovering, Developing, Maturing, Mature, Spawning and Spent). The cyclic changes in GSI indices are considered as further a proof that the spawning season is extending from November (8.88%) to March with value (0.62%). Sex steroids hormones play a role in controlling the maturation cycle in teleost especially during spawning times, which altered by environmental or hormonal manipulation. 17β - Estradiol levels in female L. carinata ranged from maximum value 0.199 ± 0.087 ng/ml recorded in maturing stage to 0.102 ± 0.016 ng/ml ml in December 2012. The lowest value of testosterone was observed in February with value (17.4 ± 1.054) in spawning and spent stages of maturity.
1 Introduction
Liza carinata belongs to family Mugilidae. That consists around the world of more than 72 species from 17 fish genera (Nelson, 2006). Liza carinata is one of the commercially important fish species in Suez Bay and Egypt, although it has a lower growth rate, fetches a higher market price compared to the other mullet in Egypt because of its highly appreciated taste. Numerous investigations have been conducted on Mullet species such as (Abou-Seedo and Stephen, 2004) studied the Liza klunzingeri reproductive cycle, (El-Halfawy et al., 2007) studied the reproductive biology and histological character of L.ramada, (Liu et al., 2009) investigated the histological demography of Mugil cephalus, (Hakimelahi et al., 2011) studied the reproductive biology of female Liza klunzingeri and (El-Ganainy et al., 2014) studied the reproductive biology of L.carinata in Suez bay.
The oogenesis is a very dynamic process in the ovaries, in which the oocyte passes through various phases of the development that are very similar in different fish species. The fish oocyte development can be divided into oocyte growth and oocyte maturation. Vitellogenesis plays an important role in the oocyte growth. Germinal vesicles migrate and breakdown, coalescence of lipid droplets and yolk globules, and release of the 1st polar body are the characteristic event in the process of maturation (Weltzein et al., 2002).
Histological investigations of female gonads have been conducted on Mullet species (Sung et al., 2004; Assem et al., 2008; Yelghi et al., 2012) studied the development of ovary and oocyte growth in Mugil cephalus. (Mônica Rocha et al., 2011) studied the ovarian development of Mugil curema. (Erdinç et al., 2011) demonstrated the histological changes in Liza abu. Although Egypt recorded as a first mullet aquaculture production it reached (4.43, 5.95, 5.93) percent in (2009-2010-2011) respectively (El Gamal, 2013), it suffering from deficiency in L.carinata production and fries number due to illegal fishing and collection of fries which require a solution to save stock number, by having deep knowledge of the biological, reproductive cycle and histological features of the target species to help induced spawning of it, the previous studies tried to study the biological and histological reproductive characters of L.carinata in the same study area (Mahmoud, 1997; El-Ganainy et al., 2014) but the present study caring about the steroids hormones concentration which represent a complete knowledge of reproductive and hormonal pattern of L.carinata help to give a solid ground for further induced spawning studies of this species.
Study on annual reproductive cycle of plasma sex steroid hormones and gonad maturity of fish is crucial in understanding spawning pattern in fish (Lee and Yang, 2002; Manosroi et al., 2003). Sex steroids are responsible for gonad maturation. Influence of sex steroid hormones like testosterone, and 17β - estradiol in gonad maturation need to more - emphasized (Lubzens et al., 2010; Schulz et al., 2010). Synthesis of vitellogenin and increase in ovarian size during final oocyte maturation is controlled by 17b - estradiol which is directly related to gonadosomatic index (Tyler et al., 1991; Sabet et al., 2009; Heidari et al., 2010; Adebiyii et al., 2013).
This study aims to investigate the annual reproductive cycle of females of Liza carinata during processes of formation eggs (oogenesis) and the maturity stages of ovary respectively in addition to the description of the annual reproductive cycle and GSI in relation to sex hormonal profiles of testosterone and 17β - estradiol during the spawning period throughout the year.
2. Materials and Methods
2.1 Study area
Suez Bay or (Bahr el Qulzum) extending about 4.5 miles south from its head is entered between Ras - El Adabia and Ras - Misalla (NGIA, 2007). Suez Bay is shallow extension on Gulf of Suez, roughly elliptic in shape, with its major axis in northeast and southwest direction and extends from 29ᵒ51′ to 30ᵒN. The bay is connected to Suez Gulf through the south - eastern side and connected to Suez Canal through the north - eastern side. City of Suez occupies the northern part of the bay.
2.2 Sampling of fish and histological investigation
718 samples of Liza carinata collected from Suez Bay and transferred immediately to the laboratory. The gonads were removed and weighted to the nearest 0.1 g. Gonadosomatic index (GSI) of this fish was expressed a percentage of gonad weight (GW) to gutted body weight (BW). Thus: GSI = GW. BW-1. 100%.
Fish were anaesthetized and blood samples were taken from the caudal vessels by using heparinized disposable syringes. Sample was centrifuged for 10 min at 3000 rpm and the plasma was stored at -45oC until steroid analysis. Plasma levels of (E 2) and (T) were measured by radioimmunoassay using the procedure described by (Rinchard et al., 1993). The test of profile was done in duplicate.
The fresh gonads were fixed immediately in aqueous Bouin solution. Then gonads were transferred to 70% ethyl alcohol until used. Samples were embedded in paraffin wax at 57oC. Transever sections of about 5-7 μm from female reproductive organs were made using automatic microtome and stained with haematoxylin and eosin. The stained sections were examined under Leica DM500 Microscope equipped with camera.
2.3 Statistical analysis
The average of data among treatments was analyzed by one way ANOVA followed by Tukey test using SPSS V 13. Results are presented as means ± standard error of the mean (SEM).
3 Results
3.1 Oogenesis of Liza Carinata
I – First growth phase
1 Chromatin –Nucleolus stage:
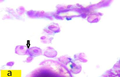
Oogonia are spherical with diameter 44.5 µm to less than 45.5 µm, having large nucleous with diameter from 10 µm to 11 µm. (Figure a) ovary which fill of oogonia represented the Immature stage which found from February till August (Figure 1).
|
Figure a Cross section of ovaries of Liza carinata showing Chromatin-Nucleolus stage stained with eosin and hematoxilin (100X), arrow showing oogonia |
|
Figure 1 Cross section of ovary showing Immature stage of Liza carinata stained with eosin and hematoxilin (10X) |
2 Perinucleolus stage:
(i) Early Perinucleolus stage:
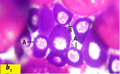
Oocyte rounded with diameter ranged from 22.5 µm to less than 38 µm. The nucleus with diameter ranged from 16 µm to 28 µm (Figure b1).
|
Figure b1 Cross section of ovaries of Liza carinata showing Early Perinucleolus Stage stained with eosin and hematoxilin (100X) Note: A showing early Perinucleolus Stage and B showing late Perinucleolus Stage |
(ii) Late Perinucleolus stage:
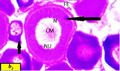
Oocyte divided into two sizes, the smallest size ranged from 92 µm to 100 µm in diameter with nucleus ranged from 55 µm to 70 µm in diameter.
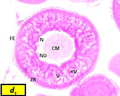
The diameter of the largest oocyte ranged from 101 µm to 116 µm with nucleus ranged from 60 µm to 70 µm in diameter (Figure b2).
|
Figure b2 Cross section of ovaries of Liza carinata left arrow showing Late Perinucleolus Stage stained with eosin and hematoxilin (40X) Note: (N) nucleus, (NU) nucleoli, (CM) chromatine material, (FE) follicular epithelium, right arrow showing yolk nucleous |
Ovary with early and late prinucleolus stage found at the end of spawning season from March to September to represent Immature & Recovering stage (Figure 2).
|
Figure 2 Cross section of ovary showing Immature & Recovering stage of Liza carinata stained with eosin and hematoxilin (10 X) |
II – Second growth phase
1 Vacuolization stage (yolk vesicle stage):
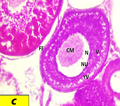
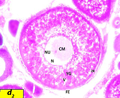
Oocytes are about 425 µm to 439 µm in diameter. The nucleus becomes larger with diameter from 75 µm to 170 µm, zona radiata with mean thickness of 16 µm (Figure c).
|
Figure c Cross section of ovaries of Liza carinata showing Vacuolization Stage stained with eosin and hematoxilin (40X) Note: (N) nucleus, (NU) nucleoli, (CM) chromatine material, (YV) yolk vesicles, (V) vacuoles, (FE) follicular epithelium |
Presence of yolk vesicle in the ovary indicated that the Developing stage is started and this is observed throughout the investigation months especially in September and October (Figure 3).
|
Figure 3 Cross section of ovary showing Developing stage of Liza carinata stained with eosin and hematoxilin (10 X) |
2 Yolk stages:
(i) Primary yolk stage:
Oocyte become greater ranged from 576 µm to 670 µm in diameter, characterized by presence of yolk vesicle layers and nucleus mean diameter became 173 µm zona radiata increases in thickness to be 31.9 µm (Figure d1).
|
Figure d1 Cross section of ovaries of Liza carinata showing Primary Yolk Stage stained with eosin and hematoxilin (40X) Note: (N) nucleus, (NU) nucleoli, (CM) chromatine material, (YV) yolk vesicles, (V) vacuoles, (FE) follicular epithelium, (ZR) zona radiata |
(i) Secondary yolk stage:
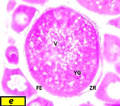
Oocyte become much larger with diameter ranged from 646 µm to 715 µm, characterized by presence of yolk globules, and irregularly nucleus shape with average diameter 123 µm zona radiata increases in width to an average of 30 µm (Figure d2).
|
Figure d2 Cross section of ovaries of Liza carinata showing Secondary Yolk Stage stained with eosin and hematoxilin (40X) Note: (N) nucleus, (NU) nucleoli, (CM) chromatine material, (YV) yolk vesicles, (YG) yolk globule, (V) vacuoles, (FE) Follicular epithelium, (ZR) zona radiata |
(ii) Tertiary yolk stage:
The diameter of oocyte generally greater than 650 µm and less than 766 µm with presence of yolk globules in much size and number, the nucleus mean diameter became 88 µm and zona radiata varies in thickness with average about 39 µm in width (Figure d3).
|
Figure d3 Cross section of ovaries of Liza carinata showing Tertiary Yolk Stage stained with eosin and hematoxilin (40X) Note: (N) nucleus, (NU) nucleoli, (CM) chromatine material, (YG) yolk globule, (V) vacuoles, (FE) follicular epithelium, (ZR) zona radiata |
The presence of oocytes from all yolky stages of development (primary, secondary and tertiary yolk globule) in the ovary found from October to November and showed the Maturing stage of ovary (Figure 4).
|
Figure 4 Cross section of ovary showing Maturing stage of Liza carinata stained with eosin and hematoxilin (10X) |
3 Ripe stage:
The egg are approximately from 925 µm to 933 µm in diameter and yolk appears to fill the interior of the oocyte, the nucleous migrated to the animal pole, zona radiata varied in thickness from about 22.5 µm at the vegetative pole to 36 µm at the animal pole (Figure e).
|
Figure e Cross section of ovaries of Liza carinata showing Ripe Stage of oocyte stained with eosin and hematoxilin (40X) Note: (YG) yolk globule, (V) vacuoles, (FE) follicular epithelium, (ZR) zona radiata |
Ovary with ripe eggs represented the Mature stage and this stage found from November to December (Figure 5).
|
Figure 5 Cross section of ovary showing Mature stage of Liza carinata stained with eosin and hematoxilin (10X) |
4 Spawning stage:
Ripe or mature ova are spawning, the ovarian membrane is thin and wrinkled than the normal one and the ovarian cavity appears large. This stage found from November to March (Figure 6).
|
Figure 6 Cross section of ovary showing Spawning stage of Liza carinata stained with eosin and hematoxilin (10X) |
5 Spent stage:
Spent ovary contain empty and rupture follicles at different phases some of them at resting phase representing few oogonia, many perinucleolus oocyte and atretic oocytes (oocytes can’t be spawned due to unfavorable conditions) and small oocytes are found, which are recruitment stock eggs in spent ovary unabsorbed and give rise to mature eggs during the following season. The ovarian wall is folded. This stage found from January to April (Figure 7).
|
Figure 7 Cross section of ovary showing Spent stage of Liza carinata stained with eosin and hematoxilin (10X) |
3.2 Gonadosomatic index (Reproductive period)
Monthly variations in GSI of female L. carinata were quite apparent (Figure 8). Considerable increase of GSI value was recorded from November (7.05% ± 0.729) followed by maximum increase in December (8.88 % ± 0.867), a slight decrease occurred in January followed by a considerable drop in March (0.62 % ± 0.179) and reach minimum levels in May (0.397% ± 0.047). From September onwards the values of GSI increased. These cyclic changes in GSI indices are considered as further a proof that the spawning season is extending from November to March (Figure 8).
|
Figure 8 Mean gonadosomatic index (GSI) of female L.carinata in Suez bay |
3.3 Sex Steriod Hormones
17β - Estradiol levels in female L. carinata ranged from maximum value 0.199 ± 0.087 ng / ml recorded in November 2012 to 0.102 ± 0.016 ng / ml ml in December 2012. Estradiol levels were low from March but afterward they increased significantly in October 2013 (Figure 9).
|
Figure 9 Monthly changes in concentration of 17β-Estradiol, Testosterone levels and GSI of female L.carinata in Suez bay |
Testosterone levels in female L. carinata ranged from 29.7.5 ± 4.922 ng / ml to 26.9 ± 2.807 ng / ml in November 2012 to October 2013 (Figure 9). The lowest value of testosterone was observed in February 2013 (17.4 ± 1.054) in spawning and spent stages of maturity. Increasing of testosterone levels was recorded as long as the maturation of ovary increase to reach its highest value in December 2012 (30.5 ± 2.037 ng / ml) where mature stage of ovaries (Table 1).
|
Table 1 Monthly concentration of 17β – Estradiol, Testosterone levels and GSI of female L.carinata in Suez bay |
4 Discussions
The histological studies demonstrated that there are two developmental phases of the oocyte namely, the primary growth phase and secondary growth phase. Also, these two phases were investigated by many authors (EL-Gharabawy, 1996). The role of the follicle cells in the oocytes is to form an active part in the transfer of proteins and other nutrients from the blood to the developing egg as reported by (Zaki and El-Gharabawy, 1991; El-Gamal, 2003; El-Halfawy et al., 2007).
First phase of oogenesis in Liza carinata is similar to the pre - maturation period Liza ramada of (Zaki and EL-Gharabawy, 1991). This phase correspond to the pre - vitellogenesis stage in red mullet (Mullus barbatus barbatus) of (Lambros et al., 2014). Yolk nucleus appeared at the end of late peri - nucleolus stage similar result was indicated by (Sung et al., 2004).
Second phase of oogenesis in Liza carinata corresponds to the vacuolization period in M. cephalus of (Assem et al., 2008). The beginning of yolk deposition is an indication of the phase of vitellogenesis (El-Gamal, 2003; Ramadan, 2010), the trophoplasmic growth period (Latif and Saady, 1973), the primary, secondary and tertiary yolk stage and the yolk formation stage (Zaki and EL-Garabawy, 1991; Ramadan, 2010). At the ripening of oocyte in Liza ramada, the migration of the nucleus to animal pole occurs. The yolk deposition in the oocytes of the present species shows the same feature as described by other authors (Zaki et al., 1986; Zaki, 1989; Zaki and EL-Garabawy, 1991).
These results are concomitant with the previsous studies performed on Caranx crysos and Pagellus erythrinus by (Assem, 2000; Assem, 2003) and with (Abou-Seedo and Stephen, 2004) for L.klunzingeri, and with some different from (Mahmoud, 1997) that detected the immature ovary month later from June to August in the same species.
In this study spawning season of female Liza carinata detected from November to March and this result agreed with (EL-Boray, 1993; Hakimelahi et al., 2011) for the same species but also with (Moacir et al., 2011) in another species M. curema in northeastern region in Brazil. Liza carinata spawning season in Khuzestan coastel waters was reported in December to March by (Hashemi et al., 2013), while the spawning period of Liza subviridis in Indian was recorded into two period from September to October and from February to March (Rahman et al., 2015). Variation in the spawning season for Liza carinata may be a result of environmental or population related factors (Stoumbound et al., 1994) or may vary from species to species, differing from one population to another population of the same species and may vary year after year within the same population (Adams, 1980; Hashemi et al., 2013). Based on these results, all maturity stages of Liza carinata observed for the population of Suez Bay, this reflects that Suez Bay is the spawning location for this species.
Monthly changes in the concentrations of circulating sex hormones and their importance for reproduction have been reported for several species of teleost. Studies have shown that annual fluctuations of hormones related to reproductive, feeding and growth cycles in fishes. Annual rhythm of hormones closely related to factors such as temperature, environment, species of fish, length of day and gonadal sex steroids (Pavlidis et al., 2000).
In a variety of species, the level of serum E2 begins to increase in accordance with the appearance of active vitellogenic oocytes, and reaches the highest levels at tertiary yolk stage oocyte in the ovary, and sharply declines in fish with postvitellogenic and atretic ovaries. (Smith and Haley, 1988; Silvers et al., 1993; Kumar et al., 2015) reported an increase in plasma E2 levels once spawning commences and remains high throughout the period of oocyte growth.
(Sen et al., 2002) reported that concentration of plasma testosterone T in Indian major carp Labeo labeo rohita is expected to be high when it is no longer needed for aromatization, while T levels during postvitellogenic stage exhibited a quick decline in this fish, coinciding with the fall of plasma E 2 concentration. A sudden drop in the plasma E 2 level in Labeo rohita from vitellogenic to postvitellogenic stage may be explained in terms of switching off the aromatise (CYP19) activity as the oocytes progressed to maturation. Almost a similar profile of E 2 has been reported during the transition from vitellogenic to maturational stage in rainbow trout (Fostier et al., 1983). This drop in circulatory E 2 levels probably reduces the intensity of sex steroid feedback, allowing the occurrence of hypothalamus - mediated gonadotropnes surge, which is required for the development of oocyte maturational competence. Increments in ovarian gonadosomatic index and oocyte development are associated with changes in 17β - estradiol levels in circulation (Lee and Yang, 2002).
The slight increase of testosterone T levels during oocyte development may be related to its role as precursor of E 2 synthesis. At high concentration, T might also be involved in hepatic vitellogenin synthesis (Rinchard et al., 1993). The sudden peak was measured when most fish were in final maturation (stage V), an effect of the release of testosterone T into the plasma when this was no longer needed for aromatization. This acute rise in testosterone indicates that oocytes are fully mature and ready to ovulate (Kobayashi et al., 1989; Adebiyii et al., 2013).
This result clarifies that sexual hormone before maturity has fluctuation and affect by different seasonal and environmental condition (Allen and Joseph, 2006).
It is known that the role of sex steroids in controlling the maturation cycle in teleost especially during spawning times is altered by environmental or hormonal manipulation, and this has both theoretical and practical relevance (Flammarion et al., 2000; Sehafii, 2014). Based on the results of this study seem, sex steroid hormones during spawning are changed, and the change of seasons and the environment is very effective.
5 Conclusions
This study cared about studying the reproductive pattern of female of Liza carinata from Suez Bay which represented a good spawning zone for this species, from the previous results spawning season considered as a long winter spawning season so over - fishing in this period affected the population number, from this point management should regulate fishing in this period to save fish-recruiting number and maintain the species abundance.
Abou-Seedo F.S. and Stephen D., 2004, Reproduction cycle in the male and female grey mullet, Liza Klunzingeri in the Kuwaiti Waters of the Arabian Gulf.Cybium, 28(2): 97-104
Adams P., 1980, Life history patters in marine fishes and their consequences for fisheries management, Fish Bull., 78: 1-12
Adebiyii F.A., Siraj S.S., Harmin S.A., and Christianus A., 2013, Plasma sex steroid hormonal profile and gonad histologyduring the annual reproductive cycle of river catfish Hemibagrus nemurus (Valenciennes, 1840) in captivity, Fish Physiol. Biochem., 39:547–557
https://doi.org/10.1007/s10695-012-9718-x
PMid:23010937
Allen J.P. and Joseph J.C., 2006, Age/size effects on juvenile green sturgeon, Acipenser medirostris, oxygen consumption, growth, and osmoregulation in saline environments, Environ. Biol. Fish., 123-142
Assem S.S., 2000, The reproductive biology of pelgic Carangid female Caranx crysos from the Egyptian Mediterranean Sea, J. Egypt. Ger. Soc. Zoo, 31(C): 195- 215
Assem S.S., 2003, The reproductive biology and the histological and ultrastructure characteristic of ovaries of female pelagic fish Pagellus erythrinus from the Egyptian Mediterranean water, J. Egypt Ger. Soc. Zool., 42 C: 77- 103
Assem S.S., El-Dahhar A., and Mourad M., 2008, Reproductive biology (histological & ultrastructure) and biochemical studies in ovaries in of Mugil cephalus from Mediterranean water, Arabian, J. Aqua. Soc., 3(1): 33-58
EL-Boray K.F., 1993, Reproduction biology and physiology characters of Mugil seheli in Suez Bay, M. Sc., Thesis, Faculty of scince., Tanta University
El-Gamal A., 2003, Histochemical studies on oogenesis of common Carp, Cyprinus carpio (L). Bull. Nat. Inst. Oceanogr. and Fish, ARE, 29, 153-176
El Gamal A., 2013, Report in Development and outlook of Egyptian aquaculture
El-Ganainy A.A., Abd El-Rahman F.A., Rizkalla W., El-Shabaka H.A., and Abo-Mesalem M.E., 2014, Age, growth and reproductive biology of the keeled mullet Liza carinata from the Suez Bay, Red Sea, Egypt, Egypt. J. Aquat. Biol. & Fish., 18(4): 1 – 8
https://doi.org/10.1007/s10695-012-9718-x
EL-Gharabawy M.M., 1996, Histology of ovarian changes during the reproductive cycle of Lithognathus mormyrus (Teleostei ; Sparidae ), J. Egypt. Ger. Soc. Zoo., 19(A): 97 -115
El–Halfawy M.M., Ramadan A.M., and Mamoud W.F., 2007, Reproductive biology and histological studies of the grey mullet, Liza ramada (Risso,1826)in the lake Timsah, Suez Canal.Egypt. J. Aquatic. Res., 33: 234:240
Erdinç S., Zafer D., Faruk A., Ramazan, Ş., and Hasan H., 2011, Reproductive characteristics of mullet (Liza abu H., 1843) ( Pisces, Mugilidae) in the Atatürk Dam Lake, Southeastern Turkey, Turk. J. Fish. & Aqua. Sci., 11: 07-13
Flammarion P., 2000, Induction of fish vitellogenin in testicular structure: preliminary results of estrogenic effects in chub, Ecotoxicol. J., 9: 127-135
https://doi.org/10.1023/A:1008984616206
Fostier A., Jalabert B., Billard R., Breton B., and Zohar Y., 1983, The gonadal steriods. In: Hoar, W.S., Randall, D. J & Donaldson, E. M. (eds.) Fish physiology, Academic press, New york, London, IX: 277-372
PMid:6840517
Hakimelahi M., Taghavi M., Kamrani E., Ghodrati S., and Vahabnezhad A., 2011, Female reproductive of the Klunzinger′s Mullet (Liza klunzingeri) in the Persian Gulf and the Oman Sea, J. Persian Gulf. Mar. Sci., 2(6): 21 -28
Hashemi S., Kashi M., and Safikhani H., 2013, Growth parameter, Length-Weight relationship and quality coefficient of klunzingeri Mullet (Liza klunzingeri (Day, 1888)) in the Coastal of Khuzestan (Northwest of Persian Gulf), J. Nov. Appl. Sci., 2(3): 60-64
Heidari B., Roozati S.A., and Yavari L., 2010, Changes in plasma levels of steroid hormones during oocyte develoment of caspian kutum (Rutilus frisii kutum), Anim Reprod., 7: 373-381
Kobayashi K., Cohen S., and Yoshida T., 1989, Characterization of the antigen-reactive T cell line mediating in vivo delayed type hypersensitivity established by antigen-induced interleukin 2. Pubmed., 4(3): 163-79
Kumar P., Arasu A., Kialasam M., Sukumarran K., Subburi R., Tyagrai G., and Nataraian M., 2015, Gonadal development and steroid hormone profile of wild caught grey mullet (Mugil cephalus), Biol. Rhythm res., 46: 601-610
Latif A.F.A. and Saady B.E., 1973, Oogenesis in the Nile Bolti Tilapia nilotica L, Bull. Ins.Ocean. Fish, 3: 183-202
Lee W.K. and Yang S.W., 2002, Relationship between ovarian development and serum levels of gonadal steroid hormones and induction of oocyte maturation and ovulation in thecultured female Korean spotted sea bassLateolabrax maculatus (Jeom-nongoeo), Aquacult. J., 207: 169–183
https://doi.org/10.1016/S0044-8486(01)00728-1
Liu J., Brown C.L., and Yang T., 2009, Population genetic structure and historical demography of grey mullet, Mugil cephalus, along the coast of china, inferred by analysis of the mitochondrial control region, Biochem. Systematics and Ecol., 37(5): 556-566
https://doi.org/10.1016/j.bse.2009.09.002
Lubzens E., Young G., Bobe J., and Cerda J., 2010, Oogenesis in teleost: how fish eggs are formed, Gen. Comp Endocrinol., 165:367–389
https://doi.org/10.1016/j.ygcen.2009.05.022
PMid:19505465
Mahmoud W.F., 1997, Reproductive and physiological characters of Mugil seheli in fish farms, M. Sc. Thesis, Faculty of Science, Suez Canal University
Manosroi A., Meng-Umphan K., and Manosroi J., 2003, Annual sex hormonal profiles, gonad development and age determi-nation of the Mekong giant catfish (Pangasianodon gigas, Chevey), Aquac Res, 34: 1379–1385
https://doi.org/10.1111/j.1365-2109.2003.00955.x
Moacir F.O., Eudriano F.D., Fύlvio A.D., and Jorge E.L., 2011, Some aspect of the biology of white mullet, Mugil curema (Osteichthyes, Mugilidae), in the northeastern region, Brazil, Pan –American J. Aqua. Sci., 6(2): 138 – 147
Mônica Rocha O., Eudriano Florêncio S.C., and Sathyabama Chellappa, 2011, Ovarian development and reproductive period of white mullet, Mugil curemain the coastal water of northern Brazil, Anim. Biol. J., 2(4): 199-212
Nelson J.S., 2006, Fishes of the world, 4th Ed. New York, Wiley, 601
NGIA, 2007, Sailing Directions – Enroute by National Geospatial –Intelligence Agency, Book (Red Sea and the Persian Gulf, Thirteen Edition, P 16), Sector 1 .Egypt – The Suez Canal and Suez Bay
Pavlidis M., Greenwood L., Mourot B., Kokkari C., and Le Menn F., 2000, Seasonal variations and maturity stages in relation to differences in serum levels of gonadal steroids, vitellogenin, and thyroid hormones in the common dentex (Dentex dentex), Gen Comp Endocrinol., 118: 14-25
https://doi.org/10.1006/gcen.1999.7440
PMid:10753563
Rahman A.U., Mohanchander P.S., Layla P.S., and Ajmal Khan S., 2015, Reproductive characteristic of green back mullet, Liza subviridis (Valianciennes, 1836), from Parangipettai water (southeast coast of India), Int. J. Pure Appl. Zool., 3(3): 240-250
Ramadan A.M., 2010, Reproductive biology and histological features of female thread fin bream Nemipterus japonicus in Gulf of Suez, Egypt, Egyptian J. Aqua. Res., 36 (3): 493-500
Rinchard J., Kestemont P., Kuhn E.R., and Foster A., 1993, Seasonal changes in plasma levels of steroid hormones in an asynchronous fish the gudgeon Gobio gobio L; (Teleosti, Cyprinidae), Gen. Comp. Endocrinology, 92: 168-178
https://doi.org/10.1006/gcen.1993.1153
PMid:8282168
Sabet S.S., Reza I.M., Bagher A.F., and Saeed G., 2009, Study on sexual maturity and levels of gonad steroid hormones in female kutum Rutilus frisii kutum (Kamenskii, 1901) during spawning season from river Sefid-Rood of the Southern Caspian Sea, J Cell Anim Biol., 3: 208–215
Schulz R.W., Renato de Franca L., Jean-Jacques L., Florence L., Chiarini-Garcia H., Nobrega R.H., and Miura T., 2010, Spermatogenesis in fish, Gen. Comp. Endocrinol., 165: 390–411
https://doi.org/10.1016/j.ygcen.2009.02.013
PMid:19348807
Sehafii H.H., Khodadadi M., and Behbahani S.A., 2014, Seasonal Fluctuations of Sex Steroid Hormones in Indian Major Carp Catla Catla in Khouzestan, Iran, J. Environ. Anal Toxicol., 4: 227. doi: 10.4172/2161-0525.1000227
https://doi.org/10.4172/2161-0525.1000227
Sen U., Mukherjee D., Bhattacharyya S.P., and Mukherjee D., 2002, Seasonal changes in plasma steroid levels in Indian major carp Labeo labeo rohita: influence of homologous pituitary extract on steroid production and development of oocyte maturational competence, Gen. Comp. Endocrinol., 128: 123–134
https://doi.org/10.1016/S0016-6480(02)00060-6
Silvers C., Johan H.S., and Haux C., 1993, Isolation, immunochemical detection and observations of the instability of vitellogenin from four teleosts, J. Exper. Zool., (267) 6: 587-597
https://doi.org/10.1002/jez.1402670606
Smith C.J. and Haley S.R., 1988, In vitro stimulation and inhibition of steroid hormone release from postovulatory follicles of the tilapia, Oreochromis mossambicus, Cell & Tissue Res., 254: 439-447
https://doi.org/10.1007/BF00225817
Stoumbound M., Rosario A., and Roman A., 1994, Reproduction in an aggregation grouper, the red hind, Epinephelus gattatus, Environ. Boil .Fish, 41: 269 -289
https://doi.org/10.1007/BF02197849
Sung J.K., Young D.L., In Kyo Y., Hea J.B., Hyung B.K., and Masaki N., 2004, Reproductive Cycle of the Female Grey Mullet, Mugil cephalus , on the Coast of Jeju Island, Korea, J. Environ. Toxicol., 19(1): 73-80
Tyler C.R., Sumpter J.P., Kawauchi H., and Swanson P., 1991, Involvement of gonadotropin uptake of vitellogenin intovitellogenic oocytes of the rainbow trout Oncorhynchus mykiss, Gen. Comp. Endocrinol., 84: 291–299
https://doi.org/10.1016/0016-6480(91)90052-8
Weltzein F.A., Taranger G.L., Karlsen O., Norberg B., 2002, Spermatogenesis and related plasma androgen levels in Atlantic halibut (Hippoglossus hippoglossus, L.), Comp. Biochem. Phys., 132: 567–575
https://doi.org/10.1016/S1095-6433(02)00092-2
Yelghi S., Shirangi S.A., Ghorbani R., and Khoshbavar Rostami H.A., 2012, Annual cycle of ovarian development and sex hormones of grey mullet (Mugil cephalus) in captivity, Iranian J. Fish. Sci., 11(3): 693-703
Zaki M.I., Dowidar M.M., and Abdalla A., 1986, Reproductive biology of Clarias gariepinus in lake Manzalah, Egypt, I- Structure of the ovaries, Folia Morphologica, 36 (3): 301-306
Zaki M.I., 1989, Gametogenesis and sexual cycle of Solea solea in Lake Quarun (Egypt), Prob. Icth. Tom., 29(4): 582-588
Zaki M.I. and EL-Gharabawy M.M., 1991, Histological characters of ovaries of Mugil capito, Egypt, J. APP. Sci., 6 (6): 13-23
. PDF(1138KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Amira Salah El-Din Hefny
. Osama Ahmad Abass
. Magdy M. El-Halafway
. Mohamed Wafeek
. Mohamed A. Abu El-Regal
Related articles
. Liza carinata
. Oocyte maturation
. Oogenesis
. Gonadosomatic index
. Sex hormones
. Suez Bay
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)


