Research Report
Survival of Tropical Benthic Amphipod Grandidierella Bonnieroides Stephensen 1948 on Different Sediment Particle Size: Implications for Ecotoxicological Testing 
2. Bogor Agricultural University, Bogor, Indonesia
 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2015, Vol. 5, No. 34 doi: 10.5376/ijms.2015.05.0034
Received: 21 Mar., 2015 Accepted: 06 May, 2015 Published: 20 May, 2015
Hindarti1 et al., 2015, Survival of Tropical Benthic Amphipod Grandidierella bonnieroides Stephensen 1948 on Different Sediment Particle Size: Implications for Ecotoxicological Testing, International Journal of Marine Science, Vol.5, No.34: 1-6 (doi: 10.5376/ijms.2015.05.0034)
Effect of sediment particle size on survival of benthic amphipod Grandidierella bonnieroides Stephensn, 1948 collected in coastal waters of Indonesia was conducted in relation to the development of a standard test protocol for measuring sediment toxicity. The association of test species with sediment substrates is one of the essential factors in a sediment bioassay. The test organism should be able to inhabit various sediment types during test periods. The amphipod was exposed to four different structure of sediment substrates types: sand only (82% of particles were in the range of 63 µm – 2 mm), sand 50%: mud 50% (57% of particle size in the range of 63 µm – 2 mm and 43% of particle in the range of <63 µm), sieved mud only (sieved through 63 µm pore size), and natural mud only for 10 days without any food addition. G. bonnieroides was well associated with all test substrates. Significantly (p<0.05) reduced survival at the sand type (>80% of sand composition) was observed, when compare to the sieved mud only and the mixture of sand 50%: mud 50% types. The survival of amphipod was strongly related to total organic matter as food source in the sediment. Results of this study suggests that G. bonnieroides is suitable for the toxicity assessment of contaminated sediments that contain mostly mud. Subsequently, further comprehensive investigation on toxicological studies are needed to develop a standard sediment bioassay
Grain size is a natural confounding factor in sediment bioassay that can lead to possibly inaccurate conclusions. Grain size can add confounding effects through both its chemical and physical properties (Shang et al., 2013; Strom et al., 2011; Howari and Banat, 2001; Wang et al., 2001; Lapota, 2000). Confounding factor related to grain size is the availability of contaminants associated with the sediment. Fine grain sediments tend to be higher in clay content and contain higher levels of organic carbon (Zhao et al., 2010). High mortality has been observed in test organisms when fine sediments are mixed. The mixing of these sediments can cause disassociation of many compounds from the sediment, increasing their bioavailability and toxicity (Lawrence et al., 1997). Amphipods and worms require a certain type of substrate, with a grain size that is neither too small nor too large which can be stressful to organisms because they can interfere with their ability to burrow. Amphipods are particularly sensitive to grain size and should be exposed to sediments with compatible grain size (Re et al., 2009).
Marine sediments provide habitat, feeding and spawning for many aquatic organisms, but they also are considered both as a reservoir and as a long-termsource of toxicants (Burton, 2002). Indeed, many chemical contaminants, entering the marine environment, have a great affinity for the organic or inorganic components of sediment particles. As a result, sediments often act as a contaminant sink with substantially higher concentrations than in the overlying water, creating the potential for toxicity to benthic organisms. Sediment bioassays have proven to be a powerful tool in studying sediment-related toxicity and are recommended along with other methodologies to obtain information on the ecological impact of contaminated sediments (Morales-Caselles et al., 2008; Carr et al., 1996; Louma and Carter, 1993; Chapman and Long, 1983). Furthermore, bioassays are currently the only way to assess the potential toxicity of field-contaminated sediments (Anderson et al., 2007; Jemenez-Tenorio et al., 2007), involving the exposure of benthic organisms to field-collected sediments and the determination of resulting toxic effects. One factor that could affect the accuracy of these tests is variability of natural physicochemical characteristic of sediment (Lamberson et al., 1992). If intrinsic characteristic of a test sediment (unrelated to anthropogenic contaminants) are not ideal for test organisms, the test may produce a false-positive result (Spies, 1989). For instant, the amphipod Rhepoxynius abronius, frequently used in marine sediment toxisity test, suffocates when attempting to burrow in fine-grained sediments (DeWitt et al., 1988). Similarly, if the physicochemical charateristic of control sediment are less suitable for the test organism than the test sediment, a false-negative could result (Spies, 1989). Sediment particle size has been reported to have effects on benthic organism. Grain size effects using solid-phase bioassays have been observed by previous investigators. (Maxon et al., 1997) reported that elevated concentration of fine-grained sediment, regardless contaminant concentration in the sediment, was the best predictor for amphipod R. abronius mortality. In contrats, grain size had no significant effect on growth the polychaete Nereis (Neanthes) arenaceodentata, whereas the number of worms placed in each exposure vessel was critical (Dillon et al., 1993).
Study on sediment bioassay using amphipod in Indonesia was limited (Hindarti et al., 2010). Therefore, in this study a benthic amphipod (Grandidierella bonnieroides Stephenson 1948) was evaluated its sensitivity on a wide range of sediment particle size in order to determine their suitability as a test organism for a sediment bioassay. The objective of this study was to test the survival of amphipod on different sediment grain sizes.
2 Results
2.1 Response of Amphipod to Natural Sediment
Grain size characteristics of control and treatment sediments are summarized in Table 1. Grain size measurement, as compared by dry weight, was in proportion with nominal treatment sediments. Mud is general term of lumping together sediments consisting of a mix of clay, silt, and may contain sand (Wentworth, 1922). In this study, sieved ‘mud only’ refers to 99.7% of mud content in the sediment, while ‘natural mud', where this amphipod is indigenous, contained only 77.2% of mud content, while ‘sand only’ 81.7% sand-type sediment.
|
|
Survival of G. bonnieroides was generally associated with sediment types. The amphipod showed reduced survival at all of the sediment types compared to the control sediment (control sediment refers to natural mud only in this study and throughout the text). The mean survival of amphipods in the control sediment fraction was 84% and in each individual replicate it varied between 80% and 90% (Table 2). The survival of the amphipods in all of the treatment was normally distributed and homogeneited after Saphiro Wilks and Barletts tests. Results of ANOVA showed that the survival of the amphipods in all of the treatment sediments was significantly (p<0.05) reduced when compared to the control sediment. There was no significant different survival in sieved mud only type and the mixture of sand 50%: mud 50%.
|
|
Overlying water temperature, pH, and dissolved oxygen, measured continuosly, ranges overall the test 25-28.44°C, 7.18-8.04, 4.56-5.86 ppm, respectively.
2.2 Correlation of Organic Content and Structure of Sediment on Amphipod Survival
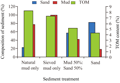
Total organic material (TOM) content in sediment was particle size dependent. The highest TOM content (8.23%) was observed at the control sediment. Exception was found in sieved mud only type of sediment (Figure 1). Although mud content in this treatment sediment was the highest (99.7%), the TOM content was found lower (7.33%) than in the control sediment.
|
|
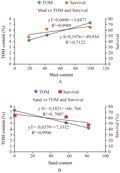
There were strong correlation occurred between mud and sand content in the sediment with TOM content and survival of amphipod. The increase of TOM content and survival amphipod were strongly related (R2>0.5) to mud content in the sediment. While the decreases were strongly related to the increase of sand type composition (Figure 2). The mean survival of amphipod was strongly related to TOM content. Amphipod survival increase with the increase of TOM content and vice versa.
|
|
3 Discussion
Amphipod mean survival in sediment controls (natural mud only) was 84% and in each individual replicate it was between 80% and 90%, This met the acceptability criteria established for this type of sediment tests (ISO, 2005). Result of this study showed a moderately sensitive to sediment grain size. The amphipod survived at more than 80% was found in muddy type of sediment (84.95% silt and 14.72% clay), while in sand type of sediment the survival was found significantly the least (44%). This suggest that the amphipod was a sensitive to sediment grain size. The amphipod has a similar sensitivity to grain size with other burrowing amphipod from Brazil, Tiburonella viscana that significantly reduced survival in laboratory-sieved sediments containing only one or two particle sizes, e.g., only medium and coarse sand, or medium sand, or fine sand, or only fines (Melo and Nipper, 2007), but less sensitive than Mandibulopho susmai from Korea (Lee et al., 2005).
Organic materials content measured in sediment was apparently affected not only by sediment particle size but also by other organic sources. Result of the study indicated high organic content found in the control sediment (natural mud only) that contain lower mud type sediment than in the in sieved mud only sediment treatment. Sieving sediment might have an effect on organic content in the sediment with loss of organic litter. These results were consistent with the data presented by (Fisher et al., 2004) that found sieving had largest changes in loss of organic concentration. These results were inconsistence with the work of (Gireeshkumar et al., 2013) that revealed a common phenomena, the accumulation of organic matter in surface sediment was mainly influenced by sediment grain size. The higher the proportion of fine sediment grain size, the higher the TOM content. Sieving sediment should be avoided because it will reduce its organic content.
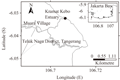
To identify the appropriate test species, we collected G. bonnieroides, which was benthic infauna, spending most of their life in sediments and also easily collectable. Basically, test organisms for sediment toxicity tests should be able to inhabit various sediment types and organic content in sediment during test periods. However, 10-d sediment incubation experiments showed that G. bonnieroides gradually survived fine-grained sediments. High survival of amphipods in sediment with a high organic content indicated that the amphipods were dependent on that food source. The work of (Pelletier et al., 2011) revealed the relationship of sediment organic carbon to grain size can be used to diagnose nutrient enrichment in estuary. The benthic amphipods collected from the western part of Jakarta Bay appear to be more sensitive to particle size than those found in Brazil (Molisani et al., 2013). Data obtained for G. bonnieroides of this study are not in agreement with those reported in that study in terms of the sensitivity of the amphipods towards a wide range of sediment particle sizes. Having found that the G. bonnieroides collected from Muara Kramat Kebo, Tangerang, Banten, Indonesia, are more sensitive to grain size, they must be considered appropriate only for sediment bioassay that has a limited range of sediment grain size, containing mostly mud type sediment.
G. bonnieroides Stephenson 1948 exhibited their sensitivity to sediment types during the experiment. The amphipods could survive in all of sediment types for the 10 d period of the test, however the survival rate corresponds to the organic content in the sediment: the highest is in the mud, the lowest is in the sand. It was proved by high correlation between mud and organic content (Figure 3). Therefore, in order to give a relevant and accurate result interpretation, it is proposed that the amphipods used only as test organism for the assessment of estuarine sediment that contains mostly mud. Other toxicological investigations should be conducted to fulfill standard test organism requirements for sediment bioassay in the assessment of contaminated sediment.
|
|
4 Materials and Methods
4.1 Preparation of test Animal
Kramat Kebo estuary is located in Tangerang, Banten Province. The estuary has muddy sediment and a relatively undisturbed environment. No significant human activities upstream affect the river. Concentrations of heavy metal contaminants in sediment were relatively low (unpublished data). Procedures of amphipod collection were as described in (Lee et al., 2005). Benthic indigenous amphipod was collected from Kramat Kebo estuary from the upper layer of sediment from the low intertidal zone using a stainless-steel spoon. Sediment containing amphipods were transferred to plastic container containing a 2- to 3-cm layer of sediment and seawater. They were transported in to the laboratory in low temperature by given ice pack into container to prevent heat. In the laboratory, the amphipods were sieved out of the sediment and sorted based on size: > 3.5 mm and < 3.5 mm lengths for adults and larvae, respectively (Hindarti, unpublished).
The individual females with black brood pouches from which eggs were obtained were initially separated into culture bins and kept for about a week during which the young were liberated. When the brood pouch turns white, it means the brood has already hatched but has not been released yet. About one or two days later, they will be released from the pouch. Their age is determined from the time they are released from the pouch. Seven-day old larvae (neonates) of amphipods are approximately 3-5 mm length and are ready for use for bioassay (USEPA 2001).
4.2 Sediment Bioassay with Benthic Amphipod
The procedures for preparing the sediment and the overlying seawater of test sediments were in accordance with Environment Canada protocol (1998). Tests were conducted in 1-L glass beakers containing a 2 cm (175 mL) layer of sediment and 775 mL of dilution water. There were four replicates prepared for the control sediment and the test sediments. Test sediments are first homogenized by careful manual mixing. Large species of material (e.g., grasses, rubber, wood, algae, etc.) and any live animals were removed at this time.
The seven-days old of amphipod were used as test animal for this study. Amphipod were incubated in sediments with four different sediment grain size compositions: natural mud only (as a control sediment) sieved mud only, a 50%:50% mixture of sand and mud, and sand only for 10 days. Sand was collected from the Pari Island beach, and mud was collected from where the amphipod lives. Both sand and mud were autoclaved to avoid any life animals prior to use in the experiment beakers. Particles bigger than 63 µm were removed by wet sieving through 63 µm screen. Five replicates beakers were applied for each sediment types and 20 individuals of test animal were incubated in each replicates. At the end of the test, all the surviving individuals are recovered using a 0.5 mm sieve and counted. Sediment was washed at least 5 times to assure that all organisms were removed. Once sieved, the rest of the sieve were washed on the clear petri dish with saline water and observed under microscope. Survival organisms were removed and counted using transfer pipette. To measure the length and weight, they were killed by placing them on ice until death. The length was measured using manual calipers and the weight was measured using a top balance. Water quality parameters including pH, temperature, and dissolved oxygen were measured in the daily basis, while salinity was measured at the beginning and the end of the tests.
4.3 Organic Matter Determination
Organic content was determined by loss on ignition (%LOI) at 600℃ for 1 hr in a blast furnace (Thomas and Bendell-Young, 1998). This procedure assumes that the difference in weight before and after ignition of the dried sediment is primarily due to loss of organic C in sediments. As sediments are dried to a constant weight prior to combustion, errors introduced due to the dewatering of clays should be relatively insignificant. Carbonates in estuarine and marine sediments may also contribute to %LOI values. However, this mineral occurs primarily as large components (e.g. broken shells) (Thomas and Bendell-Young, 1998), which were not included with collected sediment.
4.4 Data Analyses
Statistical analysis procedures recommended by U.S.EPA (1994) were adopted in this study. Sediment grain size and organic content effects on amphipod survival were assessed by analysis of Variance (ANOVA) followed by Dunnett’s multiple comparisons tests, using the statistical software package TOXSTAT release 3.3 (Gulley et al., 1991). Prior to ANOVA, the data were submitted to Saphiro Wilks and Barletts tests to verify for normality and homogeneity of variance, respectively. Linear regression analysis was used to compare mean data of two or three different treatments.
Author's Contribution
DH is a Ph.D student of the Bogor Agricultural University, Bogor. ZA, TP, ER and HSS are the promoters who are supervising in the field of Marine Biology/Ecotoxicology, Organic Chemistry, Physiology, and Marine Pollution/Ecotoxicology respectively. DH was responsible for preparing the manuscript, data analysis and sediment bioassay works.
Acknowledgement
This project was funded by the Indonesian Institute of Sciences through 2011 DIPA Research Centre for Oceanography. We thank Rachma Puspitasari, Suratno, Triyoni Purbonegoro, Eston Matondang, Adit from UNSOED, Purwokerto, for their help in the preparation of testing and data management.
References
Anderson B., Hunt J., Phillips B., Thompson B., Lowe S., Taberski K., and Carr R.S., 2007, Pattern and trends in sediment toxicity in the San Francisco Estuary. Environmental Research, 105: 141-155
http://dx.doi.org/10.1016/j.envres.2006.07.005
Ankley T., Thomas N.A., Di Toro D.M., Hansen D.J., Mahony J.D., Berry W.J., Swartz R.C. and Hoke R.A., 1994, Assessing the potential bioavailability of metals in sediment: a proposed approach. Environmental Management, 18: 331-337
http://dx.doi.org/10.1007/BF02393863
Carr R.S., Long E.R., Windom H.L., Chapman D.C., Thursby G., Sloane G.M., and Wolfe D.A., 1996, Sediment quality assessment studies of Tampa Bay, Florida. Environmental Chemistry and Toxicology, 15: 1218-1231
http://dx.doi.org/10.1002/etc.5620150730
Chapman P.M., and Long E.R., 1983, The use of bioassays as part of a comprehensive approach to marine pollution assessment. Marine Pollution Bulletin, 14: 81-84
http://dx.doi.org/10.1016/0025-326X(83)90305-3
Chariton A.A., Roach A.C., Simpson S.L., and Batley G.E., 2010, Influence of the choice of physical and chemistry variables on interpreting patterns of sediment contaminants and their relationships with estuarine macrobenthic communities. Marne and Freshwater Research 61: 1109-1122
http://dx.doi.org/10.1071/MF09263
DeWitt, T.H., G.R. Ditsworth and R.C. Swartz. 1988. Effects of natural sediment features on survival of the phoxocephalid amphipod, Rhepoxynius abronius. Marine Environmental Research, 25: 99-124
http://dx.doi.org/10.1016/0141-1136(88)90006-2
Dillon, T.M., David W.M., and Alfreda B.G., 1993, Development of a chronic sublethal Bioassay for evaluating contaminated Sediment with the marine polychaete worm Nereis (neanthes) arenaceodentata, Environmental Toxicology and Chemistry, 12: 589-605
http://dx.doi.org/10.1002/etc.5620120318
Fisher D.J., McGee B.L., Wright D.A., Yonkos L.T., Ziegler G.P., and Turley S.D., 2004, The Effects of Sieving and Spatial Variability of Estuarine Sediment Toxicity Samples on Sediment Chemistry. Archive Environmental Contamination and Toxicology, 47: 448–455
http://dx.doi.org/10.1007/s00244-003-0221-3
Gale, S.J., and Hoare, P.G., 1991, Quaternary sediments: petrographic methods for the study of unlithified rocks (Chapter 5.9). Belhaven Press, Wiley, Chichester and New York, USA
Gireeshkumar T.R., Deepulal P.M., and Chandramohanakumar N., 2013, Distribution and sources of sedimentary organic matter in a tropical estuary, south west coast of India (Cochin estuary): A baseline study. Marine Pollution Bulletin, 66: 239-245
http://dx.doi.org/10.1016/j.marpolbul.2012.10.002
Hindarti D., Puspitasari R., and Zainal A., 2010, A preliminary study on the response of amphipod Grandidierella sp. to contaminated sediment of Jakarta Bay. Marine Research in Indonesia, 35(2): 31-37
Howari, F.M., and Banat, K.M., 2001, Assessment of Fe, Zn, Cd, Hg, and Pb in the Jordan and Yarmouk river sediments in relation to their physicochemical properties and sequential extraction characterization. Water, Air and Soil Pollution, 132: 43-59
http://dx.doi.org/10.1023/A:1012062814873
ISO (International Standard Organization) 2005, ISO 16712:2005 (E): Water quality - determination of acute toxicity of marine and estuarine sediment to amphipods
Lamberson J.O., DeWitt T.H., and Swartz R.C., 1992, Assessment of sediment toxicity to marine benthos. In Burton G.A. Jr, ed, Sediment Toxicity Assessment. Lewis, Boca Raton, F.L., U.S.A., pp. 183 - 211
Lapota D., 2000, Confounding factors in sediment toxicology. (http://web.ead.anl.gov/ecorisk/issue/pdf/confound.pdf). Issue papers:1-19 Space and Naval Welfare Systems Center, San Diego
Lawrence C., Duh D., Myers J., and Pallop T., 1997, The effects of grain size and TOC on marine amphipods in whole sediment bioassays. Setac, 18th Annual Meeting, IT Corporation, 2200 Cottonil Ln, Somerset, NJ 08873
Lee J.S., Lee K.T., Kim D.H., Kim C.K., Lee J.H., Park K.H. and Park G.S., 2005, Application of indigenous benthic amphipods as sediment toxicity testing organisms. Ocean Science Journal, 40: 17-24
http://dx.doi.org/10.1007/BF03023462
Louma S.M. and Carter J.L., 1993, Understanding the toxicity of contaminants in sediment: Beyond the bioassay-based paradigm. Environmental Toxicology and Chemistry, 12: 793-796
http://dx.doi.org/10.1002/etc.5620120501
Maxon, C.L., Arthur M.B., and Douglas R.D., 1997, Sediment contaminants and biological effects in Southern California: use of a multivariate statistical approach to assess biological impact. Environmental Toxicology and Chemistry, 16(4): 775-784
http://dx.doi.org/10.1002/etc.5620160423
Melo S.L.R., and Nipper M., 2007, Sediment toxicity tests using the burrowing amphipod Tiburonella viscana (Amphipoda: Platyischnopi-
dae), Ecotoxicology and Environmental Safety, 66: 412-420
http://dx.doi.org/10.1016/j.ecoenv.2005.12.003
Molosani M.M., Costa R.N., Cunha P., de Rezende C.E., Ferreira M.I.P., and Esteves F.A., 2010, Acute Toxicity Bioassay with the Amphipod, Grandidierella bonnieroides S. After Exposure to Sediments from an Urban Estuary (Macae´ River Estuary, RJ, Brazil). Bulletin of Environmental Contamination and Toxicology, 90: 79-84
http://dx.doi.org/10.1007/s00128-012-0871-9
Morales-Caselles C., Kalman J., Micaelo C., Ferreira A.M., Vale C., Riba I., and DelValls T.A., 2008, Sediment contamination, bioavailability and toxicity of sediments affected by an acute oil spill: Four years after the sinking of the tanker Prestige (2002). Chemosphere, 71: 1207-1213
http://dx.doi.org/10.1016/j.chemosphere.2007.12.013
Pelletier M.C, Campbell D.E., Ho K.T., Burgess R.M., Audette C.D., and Detenbeck N.E., 2011, Can sediment total organic carbon and grain size be used to diagnose organic enrichment in estuaries? Environmental Toxicology and Chemistry, 30: 538-547
http://dx.doi.org/10.1002/etc.414
Rakocinski C.F., Brown S.S., Gaston G.R., Heard R.W., Walker W.W., and Summers J.K., 1997, Macrobenthic responses to natural and contaminant related gradients in northern Gulf of Mexico estuaries. Ecol Appl 7:1278–1298
http://dx.doi.org/10.1890/1051-0761(1997)007[1278:MRTNAC]2.0.CO;2
Ré A., Rosa F., Leandro S., Ana M.R., and Victor Q., 2009, Estuarine sediment acute toxicity testing with the European amphipod Corophium multisetosum Stock, 1952. Chemosphere, 76: 1323–1333
http://dx.doi.org/10.1016/j.chemosphere.2009.06.041
Shang J., Chen J., Shen Z., Wang Y., and Ruan A., 2013, Effects of varying estuarine conditions on the sorption of phenanthrene to sediment particles of Yangtze Estuary. Marine Pollution Bulletin, 76: 139–145
http://dx.doi.org/10.1016/j.marpolbul.2013.09.015
Spies R.B., 1989, Sediment bioassays, chemical contaminations and benthic ecology: New insight or just muddy water? Marine Environmental Research, 27: 73-75
http://dx.doi.org/10.1016/0141-1136(89)90001-9
Strom D., Simpson S.L., Batley G.E., and Jolley D.F., 2011, The influence of sediment particle size and organic carbon on the toxicity of copper to benthic invertebrates in oxic/suboxic surface sediment. Environmental Toxicology and Chemistry, 30: 1599–1610
http://dx.doi.org/10.1002/etc.531
Thomas C.A., and Bendell-Young L.I., 1998, Linking the sediment geochemistry of an intertidal region to metal bioavailability in the deposit feeder Macoma balthica. Marine Ecology Progress Series, 173: 197-213
http://dx.doi.org/10.3354/meps173197
US EPA (United States Environmental Protection Agency), 1994, Methods for assessing the toxicity of sediment-associated contaminants with estuarine and marine amphipods. U.S. Environment Protection Agency, Report EPA 600/R – 94/025. (Narragansett, Rhode Island 02882, USA, June 1994)
US EPA (United States Environmental Protection Agency), 2001, Method for Assessing the Chronic Toxicity of Marine and Estuarine Sediment-associated Contaminants with the Amphipod Leptocheirus plumulosus. EPA 600/R-01/020. 120 pp
Wentworth, C.K., 1922, A Scale of Grade and Class Terms for Clastic Sediments Author(s): Chester K. The Journal of Geology, 30(5): 377-392
http://dx.doi.org/10.1086/622910
Zhao X., Zheng B., Qin Y., Jiao L., Lei Zhang L., 2010, Grain size effect on PBDE and PCB concentrations in sediments from the intertidal zone of Bohai Bay, China. Chemosphere, 81: 1022-1026
http://dx.doi.org/10.1016/j.chemosphere.2010.09.007
. PDF(191KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Dwi Hindarti
. Zainal Arifin
. Tri Prartono
. Etty Riani
. Harpasis S. Sanusi
Related articles
. Amphipod
. Sediment
. Bioassay
. Particle size
. Tropical
Tools
. Email to a friend
. Post a comment