Effect of Heavy Metals (Pb, Cd, Cu) on the Growth of Sulphate Reduction associated Bacterium Clostridium bifermentans Isolated from Cochin estuary, Southwest coast of India 
 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2015, Vol. 5, No. 50 doi: 10.5376/ijms.2015.05.0050
Received: 12 Jun., 2015 Accepted: 13 Jul., 2015 Published: 01 Sep., 2015
Binish M.B., Sruthy S. and Mohan M., 2015, Effect of Heavy Metals (Pb, Cd, Cu) on the Growth of Sulphate Reduction associated Bacterium Clostridium bifermentans Isolated from Cochin estuary, Southwest coast of India, International Journal of Marine Science, 5(50): 1-5 (doi: 10.5376/ijms.2015.05.0050)
Heavy metals like Lead (Pb), Cadmium (Cd) and Copper (Cu) causes serious environmental issues. The present study investigated the tolerance and growth of Clostridium bifermentans isolated from Cochin estuary, southwest coast of India to three different heavy metals such as Pb, Cd and Cu. Growth pattern of Clostridium bifermentans in different concentrations of selected heavy metals were determined using spectrophotometer. Heavy metal tolerance (Cu, Cd, and Pb) were determined by minimal inhibitory concentration (MIC). The strain exhibited multiple heavy metal resistances and showed markedly high tolerance to cadmium and lead at minimum inhibitory concentration (MIC) of 12 µg/mL followed by copper (10 µg/mL). Presence of heavy metals significantly affected the growth of Clostridium bifermentans. Presence of lead enhanced the growth of the strain while cadmium and copper diminished the growth.
1 Introduction
The problem of pollution from heavy metals has increased worldwide in the last 3 to 4 decades. Most of the heavy metals are toxic and carcinogenic agents which cause serious environmental issues and health hazards to plants, animals and microorganisms. The heavy metal mainly enters the environment through various anthropogenic sources. Heavy metals have great tendency to bio accumulate in the environment and bio magnify in different trophic levels of food web. Higher concentration of metals in environment may kill majority of native microflora and some of them would have evolved resistance mechanism over heavy metals (Mohiuddin et al., 2011).
Higher concentration of heavy metals may exercise an inhibitory action on microorganisms by blocking essential functional groups, displacing essential metal ions or altering the active confirmations of biological molecules (Tejirian et al., 2010). Influence of heavy metals in microorganism can also cause adverse effect on its morphology, growth and biochemical activities resulting decreased biomass and diversity (Barkay et al., 1985). Therefore microbes may develop mechanism to tolerate heavy metals either by efflux, complexation or reduction of metal ions or use them as terminal electron acceptors in anaerobic respiration (Gadd, 1990). The response of microorganism to heavy metals may vary due to the concentration and availability in polluted environment. As a result heavy metal resistant bacteria can be used as biological indicators of environmental contamination. Recent studies have been reported that several microorganisms had developed multiple resistances to heavy metals (Singh et al, 2013, Jaysankar et al., 2008). It has become obvious that chromosomal and plasmid borne determinants for heavy metal resistance can transfer freely within an ecological system such as soil (Top et al., 1990).
Sulphate reducing bacteria (SRB) play a vital role in heavy metal detoxification because they enzymatically mediate the reduction of metals and forms less toxic metal sulphides (De Luca et al., 2001., Aubert et al., 1998., Macy et al., 2000). Sulphate reducing bacteria have low metal tolerance capacity, so they depend on some associated bacteria to cope with heavy metal detoxification. Clostridium species are SRB associated bacteria which plays a vital role in heavy metal detoxification (Alexandrino et al., 2014). Clostridium bifermentans is a motile, gram-positive anaerobic bacterium.
Cochin estuary, situated along the south west coast of India, is highly vulnerable to environmental pollution due to discharge from industrial, agricultural and domestic effluents. Around 0.104M m3/d of waste containing organic load are being discharged into Cochin estuary by 16 nearby industries (Balachandran et al., 2002). Total heavy metal content of sediments of Cochin estuary was higher than the average values reported from other Indian rivers (Table 1) (Mohan et al., 2012). The present study aims to determine the resistance to selected heavy metals (Pb, Cd and Cu) and growth pattern of an anaerobic bacterium Clostridium bifermentans isolated from Cochin estuary, Kerala.
2 Materials and Methods
2.1 Culture media and isolation of Clostridium bifermentans
SRB associated anaerobic bacterium Clostridium bifermentans was isolated from sediments of Cochin estuary (Figure 1) by using Sulphate API medium. 5 g sediment was directly added to 95 mL Sulphate API broth and incubated anaerobically for 7 days at 37°C. Individual colonies were isolated using pour plate method by using 0.1 ml of incubated culture in Sulphate API agar plate. Single colonies again transferred to 30 mL of Sulphate API broth and again pour plated for obtaining pure culture.
Pure culture was maintained in API broth; Yeast extract, 1 g/L., Magnesium sulphate, 0.2 mg/L., Dipotassium phosphate, 0.1 mg/L., Ferrous ammonium sulphate, 0.1 mg/L., Sodium chloride, 10 mg/L., Ascorbic acid, 0.1 mg/L supplemented with sodium lactate (Himedia laboratories Ltd., India). Pure cultures were maintained in 100 ml serum bottle containing API broth and made anaerobic by flushing 100% nitrogen gas for seven days incubation.
About 1000 µg/mL stock solutions for selected heavy metal salts were prepared from Analytical grade metal salts (CuSO4.5H2O, PbN2O6, Cd (NO3)2.4H2O) in ultrapure water, sterilized by autoclaving and stored at 4°C.
2.2 Growth studies
Growth pattern of Clostridium bifermentans was studied in 30 mL API broth supplemented with different concentrations (2 to 14 µg/mL) of heavy metals (Pb, Cd, Cu). pH 7 was maintained. Medium were inoculated with 0.3 mL young culture (48hr) of Clostridium bifermentans. Each bottle was incubated for 54 hours at 37°C. Control was maintained without adding metal in media containing bacterial culture. Anaerobic condition was maintained in all stages. Growth of Clostridium bifermentans was monitored by measuring optical density at 595 nm using UV-VIS Spectophotometer (UV MINI-1240, Shimadzu).
2.3 Heavy metal tolerance
Clostridium bifermentans susceptibilities to heavy metals (Cu, Cd, and Pb) were determined by minimal inhibitory concentration (MIC). The MIC is defined as the lowest concentration of metal that inhibited growth (Nieto, et al., 1989). 0.3mL of 48 hr incubated cultures was added to serum bottle containing 30 mL broth. Different concentrations (2 to 14 µg/mL) of heavy metal salt solutions were added to each bottle containing Sulphate API medium and anaerobically incubated for 54 hours at 37°C. A positive control consisting of a metal deficient medium inoculated with Clostridium bifermentans was maintained. A negative control consisted of broth medium without adding microorganism.
3 Results and Discussion
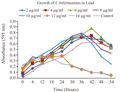
3.1 Growth studies of Clostridium bifermentansin presence of Lead
Growth pattern of Clostridium bifermentans in different concentrations of lead is given in figure 2. Control showed a clear lag, log, stationary and death phase within 54 hour incubation period. Culture incubated with 2, 4, 6, 8, 10, 12 and 14 µg/mL concentrations of lead have higher growth compared with control culture. Decreasing pattern of growth was noticed in concentrations 12 and 14 µg/mL. No visible turbidity was noted after 10 µg/mL concentration of lead.
Growth pattern of Clostridium bifermentans have higher growth rate in presence of lead but low tolerance compared with aerobic bacterium. Kafilzadeh et al., (2012) showed that aerobic bacteria bacillus spp. And corynebacterium spp. have maximum growth even at high concentration (700 µg/mLlead acetate). They observed a short pause in the growth of all bacteria when adding lead in the half of logarithmic phase growth. The same trend is noticed in the present study too.
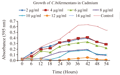
3.2 Growth studies of Clostridium bifermentans in presence of Cadmium
Control showed higher growth rate compared with other test cultures incubated with different concentrations of cadmium. Growth was not observed significantly after 10 µg/mL concentration of cadmium (Figure 3).
The studies of Mathivanan and Rajaram (2014) reported that the growth curve patterns of cadmium resistant isolates were entirely different based on the concentration of cadmium. As the added concentration of cadmium increased, the growth rates of cultures decreased. Similar results were noted in the present study, ie. decreasing growth with increasing concentration of cadmium.
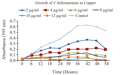
3.3 Growth studies of Clostridium bifermentans in presence of Copper
Figure 4 showed growth of Clostridium bifermentans in different concentrations of copper. Control showed higher growth within 54 hour incubation. Growth rate of Clostridium bifermentans was less in presence of copper. The bacterium exhibited no significant lag, log and stationary phase in presence of copper. But visible turbidity was noted up to 8 µg/mL concentration.
Growth rate of halophilic bacterial strain MA2 has exhibited drastic decrease in the presence of copper (Osman et al., 2012). The present study also noticed growth of Clostridium bifermentans in different concentrations of copper is decreasing compared with control.
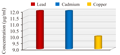
3.4 Heavy metal tolerance of Clostridium bifermentans
Clostridium bifermentans has showed maximum tolerance to different heavy metals like Cd, Pb, and Cu respectively (Figure 4). The strain showed markedly high tolerance to lead and cadmium at MIC of 12 µg/mL followed by copper (10 µg/mL). Thus from the study it is clear that the isolated strain Clostridium bifermentans has multiple resistance to different heavy metals.
Cochin estuary showed high metal contamination especially Pb, Cd, Cu, Zn, Ni and Hg. (Balachandran et al., 2005; Mohan et al., 2012, 2014). The present study indicates that heavy metal contamination in this area exerting metal tolerance to the bacterium Clostridium bifermentans. The resistant pattern of Clostridium bifermentans to lead, cadmium and copper was Pb=Cd>Cu. Under metal stress, metal resistant bacteria adapt rapidly by spread of R factors than by mutation (Bhattacherjee, et al., 1988 and Silver and Misra. 1988). Various studies have reported that in heavymetal polluted ecosystem the microorganism will develop some specific tolerance mechanism. Hence bacteria which are resistant to heavy metal may be used for bioremediation of metal polluted ecosystems (Nies., 1999, Taniguchi et al., 2000). Clostridium species have important role in bioremediation of metals and their metal precipitation may increase pH, redox potential and their metabolite production will favors SRB activity (Alexandrino et al., 2014).
4 Conclusions
Bioremediation of heavy metals by bacterial strains was greatly accepted by scientific community. In this study Clostridium bifermentans has exhibited tolerance to all three heavy metals (Pb, Cd, and Cu). An MIC of 12 µg/mL was noticed in lead and cadmium and 10 µg/mL for copper. Growth pattern of Clostridium bifermentans in different concentration of lead showed significantly high compared with control. But cadmium and copper caused for a decrease in the rate of growth at different concentration (above 2 µg/mL). It is concluded that Clostridium bifermentans have significant potential in bioremediation of lead. Thus, in future this bacterium could be used for the detoxification of lead in contaminated environment. Future studies are needed to understand the bacterial mechanism against heavy metals.
Acknowledgement
The first author acknowledges the UGC-RGNF for financial support. The authors acknowledge the facilities given by DST-FIST and DST-PURSE, Government of India. The authors also wish to acknowledge the suggestions by the anonymous reviewers in improving the quality of the manuscript.
References
Alexandrino M., Costa R., Canario A.V., Costa M.C., 2014, Clostridia initiate heavy metal bioremoval in mixed sulfidogenic cultures. Environmental Science and Technology, 48(6): 3378-85
http://dx.doi.org/10.1021/es4052044
Aubert C., Lojou E., Bianco P., Rousset M., Durand M.C., Bruschi M., Dolla A., 1998, The Desulfuromonasacetoxidanstriheme cytochrome c7 produced in Desulfovibriodesulfuricans retains its metal reductase activity. Applied Environmental Microbiology, 64 : 1308-1312
Balachandran K. K., Joseph T., Nair K. K. C., Nair M., Joseph P. S., 2002, The complex estuarine formation of six rivers (Cochin backwaters system on west coast of India)- Sources and distribution of trace metals and nutrients. APN/ SASCOM/LOICZ Regional Workshop on Assessment of Material Fluxes to the Coastal Zone in South Asia and their Impacts. Negombo, 8-11 December. Sri Lanka.
Balachandran K. K., Laluraj C. M., Nair M., Joseph T., Sheeba P., Venugopal P., 2005, Heavy metal deposition in a flow restricted, tropical estuary. Estuarine Coastal Shelf Science, 65: 361-370
http://dx.doi.org/10.1016/j.ecss.2005.06.013
Barkay T., Tripp S.C., Olson B.H., 1985, Effect of metal-rich sewage sludge application on the bacterial communities of grass lands. Applied Environmental Microbiology,46: 970-977
Bhattacherjee J.W., Pathak S.P., Gaur A., 1988, Antibiotic resistance and metal tolerance of coli form bacteria isolated from gomti river water at lucknow city. Journal of General and Applied Microbiology, 34: 391-399
http://dx.doi.org/10.2323/jgam.34.391
De Luca G., Philip D.P., Dermoun Z., Rousset M., Vermeglio,A., 2001, Reduction of technetium(VII) by Desulfovibriofructosovoransis mediated by the nickel-ironhydrogenase. Applied Environmental Microbiology, 67 : 4583-4587
http://dx.doi.org/10.1128/AEM.67.10.4583-4587.2001
Gadd G.M., 1990, In Microbial Mineral Recovery (Ehrlich, HL and Brierley, CL., Eds.) McGraw-Hill, New York, 249-275
Jaysankar D., Ramaiah N., Vardanyan L., 2008, Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Marine Biotechnology,10(4): 471-477
http://dx.doi.org/10.1007/s10126-008-9083-z
Kafilzadeh F, Afrough R, Johari H, Tahery Y, 2012, Range determination for resistance/tolerance and growth kinetic of indigenous bacteria isolated from lead contaminated soils near gas stations (Iran), European Journal of Experimental Biology, 2 (1): 62-69
Macy J.M., Santini J.M., Pauling B.V., O’Neill A.H., Sly L.I., 2000, Two new arsenate/sulfate-reducing bacteria: mechanisms of arsenate reduction. Archives of microbiology, 173: 49-57
http://dx.doi.org/10.1007/s002030050007
Mathivanan K., Rajaram R., 2014, Tolerance and biosorption of cadmium (II) ions by highly cadmium resistant bacteria isolated from industrially polluted estuarine environment., Indian Journal of Geo-Marine Sciences (IJMS).43(4)
Mohan M., Augustine T., Jayasooryan K. K., ShyleshChandran M. S., Ramasamy E. V., 2012, Fractionation of selected metals in the sediments of Cochin estuary and Periyar River, southwest coast of India. Environmentalist, 32: 383-393
http://dx.doi.org/10.1007/s10669-012-9399-0
Mohan M., Chandran M.S., Jayasooryan K.K., Ramasamy E.V., 2014, Mercury in the sediments of Vembanad Lake, western coast of India. Environmental Monitoring and Assessment, 186(6):3321-36
http://dx.doi.org/10.1007/s10661-014-3620-1
Mohiuddin K., Ogawa Y., Zakir H. M., Otomo K., and Shikazono H. W., 2011, Heavy metals contamination in water and sediments of an urban river in a developing country. International Journal of Environmental Science and Technology, 8(4): 723-736
http://dx.doi.org/10.1007/BF03326257
Nies D.H., 1999, Microbial heavy metal resistance.Applied Microbiology and Biotechnology, 51: 730-750
http://dx.doi.org/10.1007/s002530051457
Nieto J.J., Fernandez R., Castillo Marquez M. C., Ventosa A., Quesada A.E., Ruiz-berraquero F., 1989, Survey of metal tolerance in moderately halophilic eubacteria., Applied And Environmental Microbiology, 55(9):2385-239
Osman O., Tanguichi H., Ikeda K., Park P., Tanabe-Hosoi S., Nagata S.,2010, Copper-resistant halophilic bacterium isolated from the polluted Maruit Lake, Egypt. Journal of Applied Microbiology, 108(4):1459-70
http://dx.doi.org/10.1111/j.1365-2672.2009.04574.x
Silver S., Misra K.T., 1988, Plasmid-mediated heavy metal resistances. Annual Review of Microbiology, 42: 717-743
http://dx.doi.org/10.1146/annurev.mi.42.100188.003441
Singh Y.,RamtekeP.W.,Tripath A.,Shukla P.K.,2013, Isolation and Characterization of Bacillusresistantto multiple heavy metalsInt.J. Curr.Microbiol. App. Sci, 2(11): 525-530
Taniguchi J., Hemmi H., Tanahashi K., Amano N., Nakayama T., Nishino T., 2000, Zinc biosorption by a zinc-resistant bacterium, Brevibacterium sp. strain HZM-1. Applied Microbiology and Biotechnology, 54: 581-588
http://dx.doi.org/10.1007/s002530000415
Tejirian A., and Xu F., 2010, Inhibition of cellulase-catalyzed lignocellulosic hydrolysis by iron and oxidative metal ions and complexes. Applied and Environmental Microbiology, 76(23): 7673-7682
http://dx.doi.org/10.1128/AEM.01376-10
Top E., Mergeay M., Springael D., Verstraete W.,1990, Gene escape model: transfer of heavy metal resistance genes from Escherichia coli to Alcaligeneseutrophus on agar plates and in soil samples. Applied Environmental Microbiology, 56(8): 2471-2479
. PDF(388KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Binish M.B.
. Sruthy S.
. Mahesh Mohan
Related articles
. Bioremediation
. Pollution
. Metal tolerance
. Anaerobic
Tools
. Email to a friend
. Post a comment
.png)