Research Article
Assessment of Heavy Metals Pollution Using Sediments and Bivalve Brachidontes variabilis as Bioindicator in the Gulf of Suez, Egypt 
2. Chemistry Dept., Faculty of Science, Suez University, Egypt
 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2016, Vol. 6, No. 26 doi: 10.5376/ijms.2016.06.0026
Received: 18 Jun., 2016 Accepted: 08 Aug., 2016 Published: 22 Aug., 2016
El-Moselhy K.M., Saad E.M., El-Shaarway R.F., Mohamadein L.I., and Mahmoud S.A., 2016, Assessment of Heavy Metals Pollution using Sediments and Bivalve Brachidontes variabilis as Bioindicator in the Gulf of Suez, Egypt, International Journal of Marine Science, 6(26): 1-12 (doi: 10.5376/ijms.2016.06.0026)
The present work aimed to assess the quality of the coastal area of the Gulf of Suez by using sediments and bivalve Brachidontes variabilis to monitor heavy metal ions (Cd(II), Pb(II), Cu(II) and Zn(II)) at different stations along the western side of the gulf. The samples were collected twice per year (summer and winter) from seven stations representing different pollution sources. The concentration of studied heavy metal ions were determined using flame Atomic Absorption Spectrophotometer. The results showed that the heavy metal ions levels ranging from 1.34-2.60, 5.74-51.12, 3.37-57.91 and 14.69-95.96 µg/g (sediments) and 0.18-0.56, 0.53-2.54, 2.11-4.38 and 8.12-17.04 µg/g (B. variabilis) for Cd(II), Pb(II), Cu(II) and Zn(II) ions, respectively. In summer season, the high values of the most studied metals were showed, with significant difference (p < 0.05) for Pb only. The indices such as contamination factor (CF), pollution load index (PLI) and metal pollution index (MPI) were estimated in sediments and bivalve species to assess the degree of contamination from heavy metals at the different investigated stations. The obtained data indicated that the present study area was varied between low and moderate contamination with progressive decline in the quality of the investigated sites.
1 Introduction
More than half the world’s population lives within 60 km of the shoreline and this could rise to three-quarters by the year 2020. Adverse anthropogenic impacts on the coastal environment include eutrophication, organic, heavy metals, microbial pollution and oil spills. So, levels of contaminants in the marine environment are increasing continuously. In order to establish adequate coastal management programs, it is important to characterize the environment of concern chemically (Kesavan et al., 2013). The extent of contamination can be assessed by measuring pollutant concentrations in water, sediments and aquatic organisms.
Heavy metals pollution has been a hot issue in marine environmental studies for many years. Even though metals occur naturally in the environment, due to the anthropogenic inputs which originate from various human activities the concentrations have been rising (Kanakaraju et al., 2008; Lias et al., 2013). Increased coastal population, rapid urbanization, oil production, industrial and tourism development, in addition to various economic activities have created numerous environmental and ecological problems in Egyptian’s coastal areas. The western northern part of the Gulf of Suez, which is an industrial development area in Egypt, is suffered from different pollution problems. These lead to raise the pollutants such as heavy metals and hydrocarbons and consequently may affect the marine organisms.
Heavy metal pollution of marine biota is an environmental concern worldwide. Sessile benthic molluscs are used as quantitative biological indicators for monitoring chemical contaminants in marine environments. This monitoring tool was first proposed by Goldberg (1975) and launched as the "International Mussel Watch", promoting the use of bivalves as the main sentinel organisms (N.A.S., 1980). Molluscs (both bivalve and gastropod) represent organisms commonly employed as bioindicators, and used as a monitor of baseline environmental metal concentrations. The use of bivalve or gastropods molluscs looks attractive as these organisms take up metals from all environmental compartments, either from the aqueous medium or through ingestion from food and inorganic particulate material and heavily concentrate them (Phillips, 1977; Bayen et al., 2004; Yüzereroglu et al., 2010). Moreover, they are appropriate as monitors in situ because they are sedentary or sessile, available all year long and easy to collect.
The aim of the present work describes the geographical patterns of Cd, Pb, Cu and Zn distributions in the west-northern Gulf of Suez coastal waters using Brachidontes variabilis as quantitative bioindicators and surface sediments pollution. It includes the investigation of seasonal effects on metal content of the Brachidontes variabilis biomonitors. Pollution indices using this species and sediments were used to compare and assess metal pollution (Cd, Pb, Cu and Zn) at different stations in the intertidal zones of the west-northern part of the Gulf of Suez.
2 The study area
The Gulf of Suez extends for about 250 km from the Suez port to Shadwan Island. The study area extends from the Suez Bay at the north to about 30 km down the Gulf of Suez (Fig. 1). Suez Bay is located between longitude 32˚ 28` and 32˚ 34` E and latitude 29˚ 54` and 29˚ 57`N. It is a shallow extension of the Gulf of Suez, roughly elliptic in shape with its major axis in the NE- SW direction. The average length along the major axis is about 13 Km, while the average width along the minor axis is about 8.8 Km. The mean depth is about 10 m and the horizontal surface area is 77.13 Km2. The tides range varies between 80 cm at neaps and 140 cm at springs. The tidal motion is assumed to take place within all the volume of the Suez Bay due to its shallowness (Morcos, 1960; Meshal, 1970; El-Moselhy and Gabal, 2004).
3 Materials and Methods
3.1. Sampling stations
Seven stations have been selected in the western side of the northern part of the Gulf of Suez for sampling with regards to the sources of pollution in the present study area.
Station (1): Summer Palace Hotel beach (west of Port Tawfic Harbour): It is affected by tourist and fishing activities. It is a semi-closed area with a low wave motion and broad tide.
Station (2): El-Zeitiya Harbour: It is affected by refineries and shipping activities of petroleum tankers.
Station (3): El-Kabanon beach: This station is affected by the domestic effluents from the old sewage plant of the Suez City and effluent of thermo-power station. Its beach is characterized by a muddy substrate.
Station (4): Ataka Electric Power Plant: It receives the waste water from the power station as well as drainage water from the Fertilizers Company.
Station (5): Beach of the National Institute of Oceanography and Fisheries (NIOF): It receives water from the new sewage treatment plant of the Suez City, as well as the wastes of the Vegetable and Oil Company, Steel factory and the Trust textile factory.
Station (6): North of the Adabiya Harbour: It is affected by the shipping and trading operations in the harbour as well as the untreated sewage from a small nearby community.
Station (7): Sand Beach Resort shore: It is affected by tourist activities especially during summer season.
3.2 Sampling
Surface sandy/clay sediments and marine bivalves were collected twice during summer 2014 and winter 2015 from the intertidal area of the studying stations (Fig. 1). This area hosts a large part of the industrial and residential activities.
|
Figure 1 Map of the northern part of the Gulf of Suez showing the sampling sites |
Surface sediment samples were collected from the intertidal area using plastic spatula. The samples were stored in plastic bags and transferred to the laboratory, air dried at room temperature and stored in plastic bags until analysis.
In the present work, one species was selected as potential quantitative bioindicators “Brachidontes variabilis”. This species, also known as Mytilus arabicus, is a typical inhabitant of hard substrata and lives in shallow water (at sea level or just below) attached mostly in clusters to rocks and stones by its byssus. Its abundance seems to be negatively associated with wave exposure.
Bivalve species (Brachidontes variabilis) was collected from the different study stations. Organisms were collected in polyethylene bags and transferred to the laboratory in ice box, where they were identified and cleaned, then frozen at -20 ºC until analysis.
3.3 Analysis
Dried Surface sediment samples were sieved and 0.5 g of the fine fraction (< 0.063 mm) was digested according to Oregioni and Aston (1984) technique; concentrated nitric, perchloric and hydrofluoric acids were added in the ratio of 3:2:1 to the sample in Teflon vessels and left overnight. The sample was then heated to 100 ºC for about two hours, cooled, filtered and diluted to 25 ml with deionized water. Metals were determined by Flame Atomic Absorption Spectrophotometer (FAAS Perkin Elmer model AAnalyst 100); the results obtained were expressed in µg/g.
Brachidontes variabilis were thawed and rinsed in distilled water. The preparation of samples to determine concentration of heavy metals was carried out according to FAO (1976). Total soft tissues were separated from the shells, weight and digested at 100 ºC using AR conc. nitric acid in Teflon digested vessels. Replicate samples from each station were used for metal analysis; usually one individual of large animals represented a replicate, but composite samples from smaller size organisms were used for each replicate. Wet digested samples were filtered, diluted with deionized distilled water and analyzed for Cd, Pb Cu and Zn using Flame Atomic Absorption Spectrophotometer (FAAS Perkin Elmer model AAnalyst 100). The obtained results were expressed in µg/g wet weight.
De-ionized water was used to prepare all solutions and blanks. All vessels and glassware were soaked in 10% nitric acid overnight and later rinsed with distilled water. Precision of the methods was verified by analysis of replicate measurements for the studied metals in the sample of sediments and marine organism. The obtained results showed precision of 7.5 – 12.3 and 4.1 – 9.8 %, respectively, for all studied metals.
3.4 Statistical Analysis
Heavy metal data in sediments and bivalve species were subjected to analysis of variance “ANOVA” to investigate the differences between stations and seasons concentration (significant values, p≤0.05 for all analysis). Duncan's multiple range test was used to further determine the position of the variance in significant results. Statistical analysis was carried out using Statistica X software packages for windows and Origin Pro 9.
4 Results and Discussion
4.1 Heavy metals in sediments
Table (1) show the values of Cd, Pb, Cu and Zn in sediment samples collected during summer and winter from different investigated stations along the western side of the northern part of the Gulf of Suez. It can be observed that, at all sampled stations, the concentrations of Zn represent the highest value in the present study area followed by Pb(II) and Cu(II) ions, while Cd(II) ion was the lowest one. As well as Cd(II), Pb(II) and Cu(II) ions showed the highest mean values during summer season; in contrast, Zn(II) was found in winter season (Fig. 2). During summer, heavy metals concentration in sediments from Gulf of Chabahar, Oman Sea were increased markedly (Bazzi, 2014); and he suggested that increasing of nutrient availability due to upwelling during summer season enhanced phytoplankton growth followed by increasing of suspended organic matter which should be involved of heavy metals enrichment of sediments. Statistically, using ANOVA analysis, only Pb(II) ion revealed significant variation between the two studied seasons (p = 0.0426), while the other metals showed insignificant variations. Abouhend and El-Moselhy (2015) investigated seasonal variations of heavy metals in the sediments from northern Red Sea, and recorded small temporal range of metal levels with high concentrations during winter and autumn seasons.
|
Table 1 Concentration of heavy metals and values of contamination factor (CF) in sediments collected from northern part of the Gulf of Suez during 2014-2015. |
|
Figure 2 Mean concentrations of heavy metals (µg/g) in sediments of the northern part of the Gulf of Suez. |
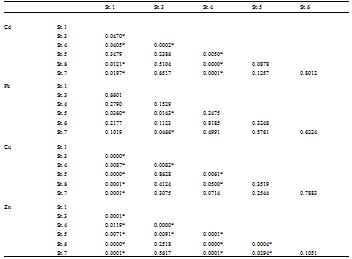
According to the local distribution of metals along the investigated area, it can recorded that Pb(II), Cu(II) and Zn(II) ions exhibited their absolute highest value (51.12, 57.91 and 95.96 µg/g, respectively) at El-Zeitiya Harbour (Station 2), with annual means of 46.93, 45.16 and 75.29 µg/g, respectively. This station is exposed to pollution from oil refineries and shipping activities of petroleum tankers in addition to discharges from neighboring industries and other human activities. El-Moselhy et al. (1999) indicated that the land based activities and ships waiting in the area are the main sources of metal pollution in the northern part of the Gulf of Suez. In this context, El-Moselhy and Gabal (2004) found that the highest values of metals were observed at stations influenced by various pollution sources such as harbours and sewage and industrial drains. Accordingly, El-Kabanon beach (Station 3) showed maximum concentration of Cd(II) ion (2.60 µg/g with annual mean of 2.46 µg/g). This station is influenced by the old sewage plant of the Suez City and effluent from thermo-power station in addition to current water from the northern area of the Suez Bay all over the year. In contrast, the lowest concentrations of Cd, Pb and Cu were recorded at Sand Beach Resort shore (Station 7), which is fare away from any pollution sources. While, Zn(II) ion revealed its lowest value at station 3. The variations of Cd(II) and Cu(II) ions within the different studied stations were significantly different (p = 0.0370 and 0.0080, respectively); while Pb(II) and Zn(II) ions showed insignificant differences (p = 0.1438 and 0.0775, respectively). According to Post-Hoc Comparisons of Means “Duncan test” (Table 2), it can be noticed that the low Cd(II) ion value in station 7 was the position responsible on the significant differences (p = 0.0063, 0.0134 and 0.0084 with St. 3, 5 and 6, respectively). In contrast, high level of Cu(II) ion in station 2 was the liable that gave significant differences with all other stations. In addition the station 2 showed significant differences than stations 3 and 7 for Pb(II) and Zn(II) ions.
|
Table 2 Duncan’s multiple range test showing significant differences of metals in sediments at different stations. |
Heavy metals in sediments of the present study area were lie within the range of those recorded in other Egyptian coastal areas and elsewhere (El-Moselhy and Gabal, 2004; Hamed and Emara, 2006; El-Moselhy and Hamed, 2006; Sany et al., 2011; Abouhend, 2013; Zaghloul, 2015 and Saad et al., 2016). By comparing the present data with the background concentrations and typical levels of metals in sediments which were 0.30, 39.0, 19.0 and 98.0 µg/g (Bryan, 1985), it can be noticed that the levels of metals in the present study showed lower concentrations, except Cd(II) ion in sediments of all stations, Pb(II) and Cu(II) ions in station 2 which were higher than the background concentration.
4.2 Heavy metals in Brachidontes variabilis
The concentrations of Cd, Pb, Cu and Zn in bivalve B. variabilis collected during summer and winter from different stations along the western side of the northern part of the Gulf of Suez are presented in Table (3). Zn was the highest recorded metal in the investigated species followed by Cu and Pb, while Cd was the lowest one. Cd, Pb and Zn exhibited their high mean values during summer season; while, Cu was found in winter season (Fig. 3). In this context, the soft tissue of bivalve Paphia undulata from Lake Timsah, Suez Canal accumulated high concentrations of heavy metals during the worm months, spring-summer (El-Moselhy and Yassien, 2005). As in sediments, Pb only showed significant temporal difference (p = 0.0198), while other studied metals recorded insignificant variations (p = 0.1007, 0.1372 and 0.7968 for Cd, Cu and Zn, respectively).
|
Table 3 Concentration of heavy metals and values of contamination factor (CF) in bivalve B. variabilis collected from northern part of the Gulf of Suez during 2014-2015. |
|
Figure 3 Mean concentrations of heavy metals (µg/g) in bivalve, Brachidontes variabilis collected from the northern part of the Gulf of Suez. |
Temporal variations in heavy metals accumulation, which were reported in mollusca species from other regions (Szefer et al., 1999; Yüzereroglu et al., 2010; Singh et al., 2012, 2013 and Rashida et al., 2015), may be attributed to different factors such as food supply for the mollusc populations and/or runoff of particulate metal to the coastal waters of the lagoon. Seasonal fluctuations of tissue metal concentrations in molluscs may be affected by various environmental (physicochemical conditions of water) and biological factors (physiological state of organism); and have been related to a great extent to seasonal changes in flesh weight during development of gonadic tissues (Cossa and Rondeau, 1985; Joiris et al., 1998; Otchere et al., 2000, 2003 and Sokolowski et al., 2004).
In respect to the spatial distribution of the studied metals in the bivalve B. variabilis, station 2 is characterized by oily sediments which suffered from pollution coming from the surrounding oil industrial area, therefore it has no any marine life appearance; accordingly, we did not found bivalve species in this station. For other stations, Cd and Zn exhibited their highest values at station 4 (0.56 and 17.04 µg/g, with an annual means of 0.40 and 16.46 µg/g, respectively), while Pb was found at station 5 (2.54 with an annual mean of 1.70 µg/g) and Cu was in station 1 (4.38 with an annual mean of 4.06 µg/g). Lowest values of the studied metals were recorded at stations 6 (0.18 µg Cd/g and 8.12 µg Zn/g), 1 (0.53 µg Pb/g) and 7 (2.11 µg Cu/g). In the present investigated bivalve, variations of Cd, Pb, Cu and Zn within the different studied stations were significantly different (p = 0.0001, 0.0424, 0.0000 and 0.0000, respectively). Post-Hoc Comparisons of Means “Duncan test” (Table 4) showed that the difference in Cd, Cu and Zn concentrations were significantly at a position of stations 1 and 4; while Pb was detected at stations 3 and 5.
|
Table 4 Duncan’s multiple range test showing significant differences of metals in bivalve B. variabilis at different stations. |
The obtained results of the studied metals in soft tissues of B. variabilis were more or less comparable with those recorded in mollusca species by Yassien (1998), El-Moselhy et al. (1999), El-Moselhy and Gabal (2004), Kesavan et al. (2013) and Sharaf and Shehata (2015). In order to the variation in metals content in the different mollusca species may be attributed to the bioavailability of each species to uptake metals from the surrounding areas. In unpolluted and mildly polluted waters, residues of the present studied metals (Cd, Pb, Cu and Zn) are typically < 3, 5, 20 and 300 µg/g, respectively, in base of wet and dry weight regardless of species (Chiu et al., 2000; Widdows et al., 2002 and Bayen et al., 2004). Accordingly, the present results were lies in the range of those recorded for unpolluted and middle polluted water.
4.3 Assessment of heavy metals contamination
In the interpretation of the present obtained data, choice of background values plays a significant contribution. Several researchers have used the average shale values or the average crustal abundance data as reference baselines for sediment samples, these values are 0.1, 14.8, 25 and 52 µg/g for Cd, Pb, Cu and Zn, respectively (Wedepohl, 1995; Loska and Danuta, 2003 and Islam et al., 2015). While for bivalve B. variabilis, the lowest mean values in the present study were used as baseline concentrations. The degree of contamination from heavy metals could be evaluated by determining the contamination factor (CF), pollution load index (PLI) and metal pollution index (MPI) from the following formulae 1, 2 and 3 [Tomlinson et al., 1980 and Usero et al., 2005].
.jpg) ......................................................... 1
......................................................... 1
Where 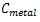 and
and 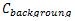 are the metal concentration and the baseline metal concentration, respectively.
are the metal concentration and the baseline metal concentration, respectively.
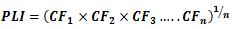 ............................................................... 2
............................................................... 2
Where n and CF are the metal number and contamination factor, respectively.
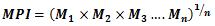 ............................................................... 3
............................................................... 3
Where n and M are the metal number and metal concentration, respectively.
The ratio of the measured concentration to natural abundance of a given metal had been proposed as the contamination factor (CF); thus, the CF values can monitor the enrichment of one given metal in sediments over a period of time. The obtained data for CF in sediments and bivalve species showed the range of 0.14 - 24.60 and 1.00 – 2.11, respectively, with high value for Cd (15.90 - 24.60) in sediments samples. Harikumar and Jisha (2010) stated that CF < 1 refers to low contamination, 1 ≥ CF ≥ 3 means moderate contamination, 3 ≥ CF ≥ 6 indicates considerable contamination, and CF > 6 indicates very high contamination. Accordingly, the present studied area was varied between low and moderate contamination regardless Cd in sediments which referred very high contamination.
The pollution level in different aquatic ecosystems was calculated using Pollution Load Index (PLI) (Tomlinson et al., 1980; Chaudhuri et al., 2007; Nomaan et al., 2012; Shams El-Din et al., 2014 and Ali et al., 2016). It represents the number of times by which the heavy metal concentration in the samples exceeds the background concentration, and gives a cumulative indication of the overall level of heavy metal toxicity in a specific site. When the values of PLI are < 1, it suggests a low level of pollution, a value of one indicates the presence of only baseline level of pollutants and values above one indicate progressive deterioration of the site and estuarine quality (Tomlinson et al., 1980). The PLI gave an evaluation of the overall toxicity status of the sample and also it is a consequence of the contribution of the studied four metals. The present study area showed PLI values between 0.87 and 3.57 (for sediments) with maximum at station 2, and minimum at station 7 and between 1.07 and 1.69 (for bivalve) with maximum at station 4, and minimum at station 3. The obtained PLI values indicating progressive decline in the quality of the present investigated sites (Table5).
|
Table 5 values of the pollution load index (PLI) and metal pollution index (MPI) in sediments and bivalve B. variabilis collected from the Gulf of Suez |
In addition to calculate the PLI values, metal pollution index (MPI) can also be used to assess the quality of the coastal areas and compare the total metal content in the different compartments of the studied area. In the present study, MPI varied from 2.77 to 23.65 (sediments) and 1.47 – 2.32 (bivalve) (Table5). MPI was previously used to evaluate the metal contamination in sediments and different marine organisms, and compare its degree between locations and within different species (Giusti et al., 1999; Hamed and Emara, 2006; El-Sikaily, 2008; Abdel-Salam and Hamdi, 2014 and Ibrahim and Abu El-Regal, 2014).
According to the calculated data resulting from contamination factor and pollution indices (PLI and MPI); it can classified the degree of contamination in the present area as following: by using sediment samples, st. 2 > st. 4 > st. 6 > st. 5 > st. 1 > st. 3 > st. 7, and by using bivalve B. variabilis, st. 4 > st. 1 > st. 5 > st. 7 > st. 6 > st. 3. The difference in contamination degree between sediments and bivalve specie at each site may be attributed to that bivalve exposure to metals not only from the sediments or from surrounding water, but also through prey consumption which in turn bioaccumulation of heavy metals in their tissues (Wang and Fisher, 1999). Molluscs as filter feeder organisms are most frequently used to monitor the pollution of coastal water by metals (Zia and Khan, 1989). Lying in the second trophic level in the aquatic ecosystem, mollusks have long been known to accumulate trace elements in aquatic ecosystems (Phillips, 1977). However, sediments are the reservoir of metals in the aquatic systems and can be used as indicator of pollution in the coastal areas, but molluscs species “B. variabilis in the present study” can easily use to assess the metal pollution and is a good bioindicator for heavy metals.
5 Conclusion
The land based activities, oil industry and the different shipping activities are the main sources of metal pollution the Gulf of Suez. The level of the studied metals (Cd, Pb, Cu and Zn) in sediment samples and bivalve B. variabilis were varied among different stations and seasons (summer and winter). In addition to, contamination factor (CF), pollution indices (PLI and MPI) of sediments and B. variabilis were used to determine the degree of pollution of heavy metals at the different stations and, which indicated that the investigated area was varied between low and moderate contamination.
In conclusion, soft tissues of bivalve Brachidontes variabilis are suitable to be used as bioindicator for heavy metals contamination in the Gulf of Suez due to its availability to regulate and accumulate elevated concentrations of different metals and it act as a watch species in the gulf and most other Egyptian water. As well as, detailed study on the bioaccumulation level of heavy metals by other molluscs species, especially bivalve and gastropod, from the Gulf of Suez would substantially provide complete information on their utility in monitoring program.
Abdel-Salam H.A., and Hamdi S.A.H., 2014, Heavy metals monitoring using commercially important crustaceans and mollusks collected from Egyptian and Saudi Arabia coasts, Animal & Veter. Sci., 2(3): 49–61. doi: 10.11648/j.avs.20140203.11
http://dx.doi.org/10.11648/j.avs.20140203.11
Abouhend A.S.A-M., 2013, Heavy metals distribution and biosorption by marine bacteria isolated from water and sediments along Hurghada, Red Sea coastline, M. Sc. Thesis, Fac. Sci., Menoufiya Univ., pp. 141.
Abouhend A.S., and El-Moselhy Kh,M., 2015, Spatial and seasonal variations of heavy metals in water and sediments at the northern Red Sea coast. Amer. J. Water Resour., 3 (3): 73-85.
Ali M.M., Ali M.L., Islam Md.S., and Rahman Md.Z., 2016, Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh, Environ. Nanotech., Monit. & Manag., 5: 27–35.
Bayen S., Thomas G., Lee H.K., and Obbard J.P., 2004, Organochlorine pesticides and heavy metals in green mussel, Perna viridis in Singapore, Water, Air, Soil Pollution, 155: 103-116.
http://dx.doi.org/10.1023/B:WATE.0000026524.99553.55
Bazzi A.O., 2014, Heavy metals in seawater, sediments and marine organisms in the Gulf of Chabahar, Oman Sea, J. Oneanogr. & Mar. Sci., 5(3): 20–29.
http://dx.doi.org/10.5897/JOMS2014.0110
Bryan G.W., 1985, Wastes in the ocean, Vol. 6 Nearshore waste disposal, Ketchum, B. H., Capuzzo, M. J., Burt, W. V., Duedall, I. W., Park, P. K. and Kester, D. R. (eds.). John Wiley Sons, New York, 41.
Chaudhuri A., Mitra M., Havrilla C., Waguespack Y., and Schwarz J., 2007, Heavy metal biomonitoring by seaweeds on the Delmarva, Peninsula, east coast of the USA, Botanica Marina, 50: 151-158.
http://dx.doi.org/10.1515/BOT.2007.018
Chiu S.T., Lam F.S., Tze W.L., Chau C.W., and Ye D.Y., 2000, Trace metals in mussels from mariculture zones, Hong Kong, Chemosphere, 41: 101–108.
http://dx.doi.org/10.1016/S0045-6535(99)00395-1
Cossa D., and Rondeau, J.G., 1985, Seasonal, geographical and size induced variability in mercury content of Mytilus edulis in an estuarine environment: a re-assessment of mercury pollution level in the Estuary and gulf of St. Lawreence, Mar. Biol., 88: 43-49.
http://dx.doi.org/10.1007/BF00393042
El-Moselhy Kh.M., and Gabal M.N., 2004, Trace metals in water, sediments and marine organisms from the northern part of the Gulf of Suez, Red Sea, J. Mar. Sys., 46: 39–46.
http://dx.doi.org/10.1016/j.jmarsys.2003.11.014
El-Moselhy Kh.M., and Hamed M.A., 2006, Impact of land-based activities on hydrographic condition and level of heavy metals in water and sediments along the Mediterranean coast of Egypt, Egypt. J. Aquat. Res., 32 (2): 63-82.
El-Moselhy Kh.M., and Yassien M., 2005, Accumulation patterns of heavy metals in venus clams, Paphia undulata (Born, 1780) and Gafrarium pectinatum (Linnaeus, 1758), from Lake Timsah, Suez Canal, Egypt, Egypt. J. Aquat. Res., 31 (1): 13-29.
El-Moselhy Kh.M., Diab A.A., Tolba M.R., and Mohamadein L.I., 1999, Levels of some heavy metals in coastal water, sediment and the limpet Patella sp. from the northern part of the Gulf of Suez (Suez Bay), Egypt. J. Aquat. Biol. & Fish., 3 (2): 69-84.
El-Sikaily A., 2008, Assessment of some heavy metals pollution in the sediments along the Egyptian Mediterranean coast, Egypt. J. Aquat. Res., 34(3): 58-71.
FAO, 1976, Manual of methods in aquatic environment research. Part 3, sampling and analysis of biological material, FAO Fish. Tech., No. 158: 124 pp.
Giusti L., Williamson A.C., and Mistry A., 1999, Biologically available trace metals in Mytilus edulis from the coast of northeast England, Environ. Internat., 25: 969–981.
http://dx.doi.org/10.1016/S0160-4120(99)00066-5
Goldberg E.D., 1975, The mussel watch - A first step in global marine monitoring, Mar. Pollut. Bull., 6: 111–125.
http://dx.doi.org/10.1016/0025-326X(75)90271-4
Hamed M.A., and Emara A.M., 2006, Marine molluscs as biomonitors for heavy metal levels in the Gulf of Suez, Red Sea, J. Mar. Sys., 60: 220–234.
http://dx.doi.org/10.1016/j.jmarsys.2005.09.007
Harikumar P.S., and Jisha T.S., 2010, Distribution pattern of trace metal pollutants in the sediments of an urban wetlands in the southwest coast of India, Int. Jour. of Eng., 2(5): 840–850.
Ibrahim N.K., and Abu El-Regal M.A., 2014, Heavy metals accumulation in marine edible molluscs, Timsah Lake, Suez Canal, Egypt, ARPN J. Sci. & Technol., 4(4): 282–287.
Islam M.S., Ahmed M.K., Habibullah-Al-Mamun M., and Hoque M.F., 2015, Preliminary assessment of heavy metal contamination in surface sediments from a river in Bangladesh, Environ. Earth Sci., 73: 1837–1848.
http://dx.doi.org/10.1007/s12665-014-3538-5
Joiris C.R.., Azokwu M.I., Otchere F.A., and Ali I.B., 1998, Mercury in the bivalve Anadara (Senilia) senilis from Ghana and Nigeria, Sci. Total Environ., 224: 181-188.
http://dx.doi.org/10.1016/S0048-9697(98)00355-6
Kanakaraju D., Ibrahim F., and Berseli M.N., 2008, Comparative study of heavy metal concentrations in razor clam (Solen regularis) in Moyan and Serpan, Sarawak, Glob. J. Environ. Res., 2 (2): 87-91.
Kesavan K., Murugan A., Venkatesan V., and Vijay Kumar B.S., 2013, Heavy metal accumulation in molluscs and sediment from Uppanar estuary, southeast coast of India, Thalassas (An International Journal of Marine Sciences), 29(2): 15–21.
Lias L., Jamil T., and Aliaa S.N., 2013, A preliminary study on heavy metal concentration in the marine bivalves Marcia marmorata species and sediments collected from the coastal area of Kuala Perlis, North of Malaysia, IOSR J. Appl. Chem., 4 (1): 48-54.
http://dx.doi.org/10.9790/5736-0414854
Loska K., and Danuta W., 2003, Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir, Chemosphere, 51: 723–733.
http://dx.doi.org/10.1016/S0045-6535(03)00187-5
Meshal A.H., 1970, Water pollution in Suez Bay, Bull. Inst. Oceanogr. & Fish., ARE., 1: 463-473.
Morcos S.A., 1960, The tidal currents in the southern part of the Suez Canal, Gen. Ass. Helsinki, Finland., 51: 307-316.
N.A.S., 1980. The International Mussel Watch. National Academy of Sciences US, Washington, 247 pp.
Nomaan M.H., Pawar R.S., and Panaskar D.B., 2012, Assessment of heavy metals in sediments from coastal Al-Hodiedah governorate, Yemen, Univ. J. Environ. Res. & Technol., 2(3): 168–173.
Oregioni B., and Aston S.R., 1984, The determination of selected trace metals in marine sediments by Flamless/Flam-atomic absorption spectrophotometery, JAEA Monaco Lab., International Report.
Otchere F.A., Joiris C., Holsbeek L., Ali I.B., and Vanderpuye C.J., 2000 Heavy metals concentration and burden in the bivalves Anadara (Senilia) senilis, Perna perna and Crassostrea tulipa from Ghana; In: 11th Annual International Conference on Heavy Metals in the Environment (J. Nriagu, ed.), Contribution number 10161. University of Michigan, School of Public Health, Ann Arbor, MI (CD-ROM).
Otchere F.A., Joiris C., and Holsbeek L., 2003, Mercury in the bivalves Anadara (Senilis) senilis, Perna perna and Crassostrea tulipa from Ghana, Sci. Total Environ., 304: 369-375.
http://dx.doi.org/10.1016/S0048-9697(02)00582-X
Phillips D.J.H., 1977, The use of biological indicator organisms to monitor trace metal pollution in marine and estuarine environments - A review, Environ. Pollut., 13: 281–317.
http://dx.doi.org/10.1016/0013-9327(77)90047-7
Rashida Q., Olufemi A., Rana M., and Abdul Rahim A., 2015, Seasonal variation in occurrence of heavy metals in Perna Viridis from Manora Channel of Karachi, Arabian Sea, Internat. J. Mar. Sci., 5 (44): 1-13.
Saad A-E.A., Emam W.M., El-Moselhy Kh.M., Abou El-Naga E.H., and Baleg A.O., 2016, Comparative study on some heavy metals in water, sediments and fish along the Suez Canal, Egypt, Inter. J. Environ, Sci. & Engineer. (IJESE), 7: 19–29.
Sany B.T., Salleh A., Sulaiman A.H., Mehdinia A., and Monazami G.H., 2011, Geochemical assessment of heavy metals concentration in surface sediment of west port, Malaysia, Internat. Schol. and Scient. Res. & Innovat., 5(8): 411–415.
Shams El-Din N.G., Mohamedein L.I., and El-Moselhy Kh.M., 2014, Seaweeds as bioindicators of heavy metals off a hot spot area on the Egyptian Mediterranean Coast during 2008–2010, Environ. Monit. Assess., 186(9): 5865–5881.
http://dx.doi.org/10.1007/s10661-014-3825-3 PMid:24844431
Sharaf H.M., and Shehata A.M., 2015 Heavy metals and hydrocarbon concentrations in water, sediments and tissue of Cyclope neritea from two sites in Suez Canal, Egypt and histopathological effects, J. Environ. Health Sci. & Engineer., 13: 14–21. DOI 10.1186/s40201-015-0171-5.
http://dx.doi.org/10.1186/s40201-015-0171-5
Singh Y.T., Krishnamoorthy M., and Thippeswamy S., 2012, Seasonal variations of Cu, Pb, Fe, Ni and Cr in the edible wedge clam, Donax faba (mollusca, bivalvia) from the Padukere beach, Karnataka, J. Theo. Exp. Biol., 8: 95–100.
Singh Y.T., Krishnamoorthy M., Hemachandra, and Thippeswamy S., 2013, Status of some heavy metals in tissues of wedge clam, Donax scortum (bivalvia: donacidae) collected from Padukere beach, Karnataka, Internat. J. Res. & Rev. in Pharm. & Appl. Sci. (IJRRPAS), 3(2): 254–267.
Sokolowski A., Bawazir A.S., and Wolowicz M., 2004, Trace metals in the brown mussel Perna perna from the coastal waters off Yemen (Gulf of Aden): How concentrations are affected by weight, sex, and seasonal cycle, Arch. Environ. Contam. & Toxicol., 46: 67–80. doi:10.1007/s00244-003-2164-0.
http://dx.doi.org/10.1007/s00244-003-2164-0
Szefer P., Wolowicz, M., Kusak A., Deslouspaoli J.-M., Czarnowski W., Frelek K., and Belzunce M.-J., 1999, Distribution of mercury and other trace metals in the cockle Cerastoderma glaucum from the Mediterranean Lagoon Etang de Thau, Arch. Environ. Contam. Toxicol., 36: 56–63.
http://dx.doi.org/10.1007/s002449900442 PMid:9828262
Tomlinson D.L., Wilson J.G., Harris C.R., and Jeffrey D.W., 1980 Problems in the assessments of heavy metal levels in estuaries and formation of a pollution index, Helgol Meeresunters, 33: 566–575.
http://dx.doi.org/10.1007/BF02414780
Usero J., Morillo J., and Gracia I., 2005, Heavy metal contamination in mollusks from the Atlantic coast of southern Spain, Chemosphere, 59: 1175-1181.
http://dx.doi.org/10.1016/j.chemosphere.2004.11.089 PMid:15833492
Wang W.X., and Fisher N.S., 1999, Delineating metal accumulation pathways for marine invertebrates, Sci. Total Environ., 237–238.
http://dx.doi.org/10.1016/s0048-9697(99)00158-8
Wedepohl K.H., 1995, The composition of the continental crust, Geochim. Cosmochim. Acta., 59: 1217–1232.
http://dx.doi.org/10.1016/0016-7037(95)00038-2
Widdows J., Donkin P., Staff F.J., Matthiessen P., Law R.J., Allen Y T., Thain J.E., Allchin C.R., and Jones B.R., 2002, Measurement of stress effects (scope for growth) and contaminants levels in mussels (Mytilus edulis) collected in the Irish Sea, Mar. Environ. Res., 53: 327–356.
http://dx.doi.org/10.1016/S0141-1136(01)00120-9
Yassien M.H., 1998, Biological and ecological studies on the pearl oyster; Pinctada radiata (Mollusca, Lamellibranchia) from the Red Sea, with special reference to its tolerance to water pollution, Ph. D. Thesis, Fac. Sci., Ain Shams Univ.
Yüzereroglu T.A., Gök G., Çogun H Y., Firat Ö., Aslanyavrusu S., Maruldalı O., and Kargin F., 2010, Heavy metals in Patella caerulea (Mollusca, Gastropoda) in polluted and non-polluted areas from the Iskenderun Gulf (Mediterranean Turkey), Environ. Monit. Assess., 167: 257–264.
http://dx.doi.org/10.1007/s10661-009-1047-x PMid:19543988
Zaghloul Gh.Y.H., 2015, Organic and Inorganic pollutants in Suez Canal, Ph. D. Thesis, Fac. Sci. (Girls Br.), Al Azhar Univ., pp. 269.
Zia S., and Khan M.A.A., 1989, Copper uptake and regulation in a copper-tolerant Deccapod carnbarus, Bartoni fabricius, Decapoda, Crustacea, Bull. Environ. Contam. Toxicol., 42: 103-110.
http://dx.doi.org/10.1007/BF01699210 PMid:2923994
. PDF(598KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Khalid El-Moselhy
. Lamiaa Mohamadein
. Eman Saad
. Reda El-Shaarway
. Safaa Mahmoud
Related articles
. Heavy Metals
. Sediments
. Brachidontes variabilis
. Gulf of Suez
. Pollution indices
. Bioindicator
Tools
. Email to a friend
. Post a comment
.png)
.jpg)
.png)
.jpg)
.jpg)
.png)