Research Article
Studies on Amyloodinium Infestation in European Seabass (Dicentrarchus labrax.) Fishes with Special Reference for Treatment 
2 Vice Dean for Education and Studient Affairs, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Egypt
 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2017, Vol. 7, No. 24 doi: 10.5376/ijms.2017.07.0024
Received: 19 May, 2017 Accepted: 14 Jun., 2017 Published: 20 Jun., 2017
Samar S.S., Zaki V.H., Ahmed G.E., and El-Khalek N.K.A., 2017, Studies on Amyloodinium infestation in European seabass (Dicentrarchus labrax.) fishes with special reference for treatment, International Journal of Marine Science, 7(24): 229-246 (doi: 10.5376/ijms.2017.07.0024)
Amyloodinium ocellatum, adinoflagellate which causes one of the most serious diseases of warm water marine aquaculture. The parasite produces a powdery or velvety appearance on infected fish, and the resulting disease is commonly referred to as “marine velvet,” velvet disease, or Amyloodiniosis. The organism is a dinoflagellate ectoparasite and has been reported in a wide range of marine and estuarine fish. It is one of a very few organisms that can infect both teleosts and elasmobranchs (Alvarez-Pellitero, 2008). This makes it a concern for public aquaria. This ectoparasite can be found on gills and skin (body and fins) of host fish. It can cause devastating disease and mortality because the organism is able to reproduce quickly when fish are crowded, especially in closed systems. This parasite has a broad host and geographic range, causing fish mortalities in tropical and temperate environments. Rapid spread of the parasite and high mortality are common in cultured fish if the organism is not recognized and treated early in the course of an outbreak. One of the most important of the ectoparasitic protozoaisIn this respect, the present study was aimed to investigate the occurrence of Amyloodiniosis among cultured European seabass (Dicentrarchus labrax L.; Moronidae; Perciformes) fishes. This work was carried out on 1065 European seabass fishes of diffrerent life stages (546 fry-222 fingerling and 78 adults) which were collected in different seasons of the year. Cultured fish were collected from different marine farms in Egypt during the period from April 2015 to April 2016 and subjected to full clinical parasitological and histopathological examination studying the environmental stressors surrounding examined fish and their association with A.ocellatum infestation Study the ecological factors affects Amyloodiniosis. As well as examining the antiseptic activity of hydrogen peroxide against the ectoparasitic protozoan A.ocellatum as a trial for treatment.
Introduction
Amyloodinium ocellatum (an ectoparasitic dinoflagellate) is one of the most important pathogenic parasites affecting the culture of marine and brackish water fish The parasite produces a powdery or velvety appearance on infected fish, and the resulting disease is commonly referred to as “marine velvet”, velvet disease, or amyloodiniosis (Noga and Levy, 2006).
Amyloodinium ocellatum is an extremely prolific and devastating ectoparasitic dinoflagellate of fish both and brackish marine water environments. The organism is a dinoflagellate ectoparasite and has been reported in a wide range of teleosts and elasmobranchs (Alvarez-Pellitero, 2008).
European Seabass (Dicentrarchus labrax L.; Moronidae; Perciformes) is a marine fish of great economic importance particularly in Mediterranean aquaculture (Zorrilla et al., 2003a). European seabass represents a major fisheries and aquaculture species in the Mediterranean, the European Atlantic coasts and North Africa (Kuhl et al., 2010). High mortality and morbidity was recorded among differential stages of cultured European seabass (Dicentrarchus labrax) caused by Amyloodinium ocellatum.
Thus, the research work carried out in this thesis depending on three main branches:
A-Provide diagnostic procedures of Amyloodiniosis applicable under field conditions.
B-Explain the histopathological changes altered by Amyloodiniosis.
C-Statistical analysis was made with the computer program (Statistical Package for Social Sciences) SPSS. Differences among groups were assessed by means of analysis of variance (ANOVA). Subsequently, significance of differences between values was tested with the LSD post-hoc test (least significant difference test) to detect particular differences between groups. Values are represented as mean ± standard error (mean ± S.E.M). Significance indicated in figures and tables by an asterisk (*) was taken as p<0.05. Statistical analysis on data that that obtained from:
1-Investigate the occurrence of Amyloodiniosis in EuropeanSeabass Dicentrarchus labrax.
2-Study the ecological factors affects Amyloodiniosis.
3-Treatement measures using for Amyloodiniosis.
1 Materials and Methods
1.1 Fish
1.1.1 Fish used for natural examination
A total number of 1065 European Sea bass (Dicentrarchus labrax) were obtained from different marine fish farms in Egypt and examined for Amyloodinium ocellatum.
1.1.2 Fish used for experimental examination
A total number of 120 D. labrax fingerlings were obtained and acclimatized in fiberglass aquaria for conduction of treatment trial of Amyloodinium ocellatum infection.
1.1.3 Aquaria and concrete pond
Dicentrarchus labrax used for natural examinations, were held in Glass aquaria (40× 50× 60 cm).
Dicentrarchus labrax fingerlings used for conduction of treatment trial of Amyloodinium ocellatum infection, were held in fiberglass tanks (1000 L).
The used fiberglass tanks and Glass aquaria were supplied with fresh seawater and aerated through air blower. Natural seawater should be with water temperature ranged at (25 ± 3°C), Dissolved Oxygen D.O 5±3 mg/L, pH 7.4-8.8, salinity at 12±3‰ salinity and supplied with air systems.
1.2 Fish sampling
European D. labrax were sampled from April 2015 to April 2016. Freshly dead or morbid fish were taken and kept in ice boxes and transported as soon as possible to fish diseases laboratory in the National institute of oceanography and fisheries.
1.3 Water sampling
They were collected in glass or plastic and transported to the laboratory in insulated coolers and examined within (24-48 h). They include water temperature, dissolved oxygen, pH, ammonia (NH3/NH4+) and salinity, water temperature, dissolved oxygen and salinity were measured at collection sites. All water quality parameters were analyzed according to (APHA, 2005).
1.4 Laboratory diagnosis of Amyloodiniosis
1.4.1 Clinical investigation and Post Mortem (PM) examination
It was performed according to Noga (2010). Fish samples with various clinical symptoms like excessive mucous, erosions on the fins, deformity on vertebral column, pale to brownish gills were brought to the laboratory.
1.4.2 Parasitological examination
Macroscopic examination
Skin surface, fins and gills were examined by naked eyes and with the help of dissecting microscope for any attached parasites, lesions or external changes.
Microscopic examinations
Direct wet mount techniques (skin scrapping: taking smear mucus cells and scales) and gill biobsy or gill clip (at which a gill filament is removed for microscopic diagnosis).
1.5 Histopathological examination
Samples (skin, fin and gills) were carefully removed from examined Dicentrarchus labrax then fixed in neutral formalin, dehydrate in ascending grades of alcohol and clear in xylene. The fixed tissues were embedded in paraffin wax and sections of five microns were caught by using Euromex Holland microtome. Sections were stained according to Harris Haematoxylin and Eosin method (Agius and Roberts, 2003). Then sections were examined and photo taken microscopically.
1.6 Treatment trials for Amyloodiniosis
1.6.1 Supplemented antiseptic and route and dose of administration
The selected antiseptic was hydrogen peroxide H2O2 20%. It was added within a dose and duration according to (Montgomery - Brock et al., 2001).
1.6.2 Bath route
Hydrogen peroxide 20% was added in two doses 100 ppm, 200 ppm in a treated plastic container with a capacity (10 liter) far from the rearing tanks and treat for aduration of exposure 30 minutes for Amyloodiniosis and the treatment was repeated after every six days.
1.6.3 Experimental design
A total of 120 Dicentrarchus labrax fingerlings were divided into four groups of 30 each and distributed equally in fiberglass tanks size (1000 L). Each treatment was performed in triplicates. Four groups of D. labrax, the first is apparently health as control (G1) and the second was the clinically infested group (G2). The third infested was immersed with H2O2 at 100 ppm/L (G3) and the fourth infested immersed with H2O2 at 200 ppm/L (G4). It was added at 100ppm, 200 ppm as water bath in 10 liter plastic container for 30 minutes, then returned to rearing tank (1000 L). The experiment was inspected daily for 14 days and the clinical signs and mortality was recorded. The data on mean mortality intensity of infection and prevalence rate were recorded in first week &second week. The mean of length & weight of used fishes were reported in Table 1, Figure 1 and Figure 2.
|
Table 1 Comparison of Length and Weight (gm) among treated groups No. (%) after (30 min.) treatment Note: Means within the same column carrying different superscripts are sig. different at P < 0.05 based on Tukey's Honestly Significant Difference (Tukey’s HSD) |
|
Figure 1 Comparison of Length among treated groups No. (%) after (30 min.) treatment |
|
Figure 2 Comparison of Weight (gm) among treated groups after (30 min.) treatment |
There was no significance difference between the length and weight in treated and non-treated groups.
1.7 Statistical analysis
Statistical analysis was made on all data that present in this study with the computer program (Statistical Package for Social Sciences) SPSS. Differences among groups were assessed by means of analysis of variance (ANOVA). Subsequently, significance of differences between values was tested with the LSD post-hoc test (least significant difference test) to detect particular differences between groups. Values are represented as mean ± standard error (mean ± S.E.M). Significance indicated in figures and tables by an asterisk (*) was taken as p<0.05.
2 Results
2.1 Clinical signs and post mortem changes
The infested European seabass Dicentrarchuslabrax with Amyloodinium ocellatum appeared distressed, emaciated, and anorexic and showed flashing behavior. Also, there were gasping of air rapid gill movement and lying on the bottom. There was rapid and mass death of fish. Also, grossly focal erosion areas were seen on the operculum and caudal tail. Deformity in vertebral column. The affected skin showed friable skin (velvet like appearance), darkened, excessive mucous secretions. Also, sometimes grossly focal erosion caudal tail. Internally the infested fish, showed pale liver enlarged spleen and fatty position on gastro intestinal tract.
2.2 Parasitological examination
2.2.1 Morphological description
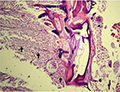
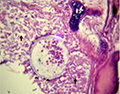
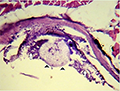
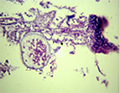
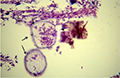
Microscopic examination of skin and gills scrapings of examined D. labrax showed, round to oval small dark brown mucoid Amyoodinium ocellatum stage measured up to 150 μm. Different developmental life stages of Amyloodinium ocellatum were seen in the gill and skin tissues as the two main inhabitant organs. The first stage was trophonts with its root - like structure (Rhizoids). They showed distended appearance with the presence of feeding stage trophonts dark brown color lodged between the gill filaments. Also, observed between the skin and fin surface the rhizoids root-like structure (that penetrates deep in to epithelium causing substantial damage to tissue at the attachment site) of the attached organelle was sometimes visible as in figures (Figure 3; Figure 7; Figure 8; Figure 10). The second was tomont stage; trophont stage feeds for several days, detaches, retracts its rhizoids and becomes tomont. Tomonts (reproductive stage) in division were occasionally observed as in (Figure 3; Figure 4; Figure 5; Figure 6; Figure 9; Figure 10; Figure 11) and a special picture of advanced stage of division of tomont stage in Figure 12.
|
Figure 3 Showing fresh mount of gill tissue heavy infested with Amyloodinium ocellatum ( trophont stage with rhizoid root) and Tomont stage( first division.) |
|
Figure 4 Showing fresh mount of gill tissue slightly infested with Amyloodinium ocellatum ( trophont stage).division |
|
Figure 5 Showing stained gill tissue infested with Amyloodinium ocellatum ( trophont stage) Geimsa X100 |
|
Figure 6 Showing fresh mount of gill tissue slightly infested with Amyloodinium ocellatum ( tomont stage) |
|
Figure 7 Showingstained fresh mountof skin infested with Amyloodinium ocellatum (Trophont stage) Geimsa X100 |
|
Figure 8 Showing stained fresh mount of skin infested with Amyloodinium ocellatum (Trophont stage) Geimsa X400 |
|
Figure 9 Showing gill tissue infested with Amyloodinium ocellatum (reproductive stage tomont) |
|
Figure 10 Showing fresh mount of gill tissue infested with Amyloodinium ocellatum (trophont stage with rhizoid root- like structure) |
|
Figure 11 Showing fresh mount of gill tissue infested heavily with Amyloodinium ocellatum (trophont and tomont stage first division (Arrowheads) |
|
Figure 12 Showing fresh mount of gill tissue infested with Amyloodinium ocellatum (advanced stage of division of tomont stage) |
2.3 Occurrence of Amyloodinium ocellatum infection
In the present study, out of the examined 1065 Dicentrarchus labrax fish, 618 (58.02%) were found to be infested with Amyloodinium ocellatum. The highest rate of infestation was recorded during spring season with Prevalence (90.10%) while the lowest was during winter season with Prevalence (30.76%). As shown in Table 2 and Figure 13.
|
Table 2 Showed Prevalence and seasonal dynamics of infestation by Amyloodinium ocellatum of European Seabass Dicentrarchus labrax. in 2015-2016 Note: G: gills; S: Skin |
|
Figure 13 Prevalence and seasonal dynamics of infestation by Amyloodinium ocellatum of Dicentrarchus labrax. |
It was necessary to study the Correlation among water quality parameters and Amyloodinium ocellatum infestation during prevalence period: as showed from (Table 3; Figure 14; Figure 15; Figure 16; Figure 17; Figure 18).
|
Table 3 Correlation among water quality parameters and parasitic infestation during prevalence period of Amyloodinium ocellatum infection Note: Correlation is significant at the 0.05 level (2-tailed). Only the pH and salinity were strong correlated with A. ocellatum infestation. Between the parasitic infestation and pH there was an inverse relationship that the result of Pearson Correlation coefficient test was (-.457) carrying a negative sign. While between the parasitic infestation and salinity there was appositive relationship that the result of Pearson Correlation coefficient test was (.473) carrying a positive sign |
|
Figure 14 Pearson Correlation between gill parasitic count and water temperature |
|
Figure 15 Pearson Correlation between gill parasitic count and water Oxygen Level |
|
Figure 16 Pearson Correlation between gill parasitic count and water PH |
.png) Figure 17 Pearson Correlation between gill parasitic water salinity |
|
Figure 18 Pearson Correlation between gill parasitic count and water ammonia level |
Figure 14 revealed that the optimum water temperature of A.ocellatum ranged between (20oC and 25oC) but the peak of infestation was recorded at water temperature (20oC). There was an inverse relationship that mean that every the water temperature increase parasitic count decrease.
Figure 15 revealed that Pearson Correlation take a straight line meaning that there was no effect and no correlation between parasitic infestation and water Oxygen Level.
Figure 16 revealed that the optimum pH of parasitic infestation ranged between (8 and 8.5) but the peak of infestation was recorded at pH 8.2. The shape of the line revealed that there was an inverse relationship that mean that every the water pH increase the parasitic count decrease.
Figure 17 revealed that the optimum salinity of parasitic infestation ranged between (16ppt and 26ppt) but the peak of infestation was recorded at salinity 26ppt. From the shape of the line that in figure there was a positive relationship that means that every the water salinity increases the parasitic count increase.
Figure 18 revealed that there was almost straight line meaning that there was a weak Correlation between parasitic count and water ammonia level.
There was direct strong significant correlation between pH levels and parasitic infestation (-.457, p=0.043). Also, there was a significant correlation between Salinity levels and parasitic infestation (473, p=0.035). There was a significant correlation between water temperature and parasitic infestation (-0.165, p=0.043). There was a weak significant correlation between ammonia levels and parasitic infestation (0.057, p=0.488).
2.4 Histopathological alterations
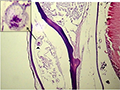
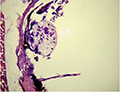
Infestations of D. labrax by Amyloodinium ocellatum are usually involve the gill as the primary site of infestation may also the skin and eyes. Thus there were histopathological changes ranged between mild infestations by low number of trophonts per gill filament and cause little alteration. But, heavy infestations by high number of trophonts can cause serious gill hyperplasia, inflammation, hemorrhage, and necrosis. As shown in (Figures 19; Figure 20; Figure 25). Trophonts were also seen inside gill arch and tissues. Concerning the mild pathological changes, Amyloodinium ocellatum trophont was attached to the skin and causing moderate hyperplasia and desquamation of the covering epithelium (Figure 23; Figure 26). On the other hand, several histpathological changes were seen in several forms as follow: Amyloodinium ocellatum trophonts causing severe hyperplasia in the gill epithelia and fusion of the secondary gill lamell Figures (Figure 21; Figure 22). Amyloodinium ocellatum trophont and its attachment site to the destructed secondary gill lamella (Figure 24; Figure 27).
|
Figure 19 showing Amyloodinium ocellatum trophont (Arrowhead) surrounded with tissue debri underneath a scale H&E X100 |
|
Figure 20 showing Amyloodinium ocellatum trophont (Arrowhead) surrounded with tissue debri underneath a scale H&E X400 |
|
Figure 21 showing Amyloodinium ocellatum trophonts (Arrowheads) causing severe hyperplasia in the gill epithelia and fusion of the secondary gill lamella (Arrow) H&E X100 |
|
Figure 22 High power of the previous figure showing Amyloodinium ocellatum trophont (Arrowheads) with the severe hyperplasia and fusion of the secondary gill lamella (Arrows) H&E X400 |
|
Figure 23 showing Amyloodinium ocellatum trophont (Arrowheads) attached to the skin causing moderate hyperplasia and desquamation of the covering epithelium (Arrows) H&E X400 |
|
Figure 24 Showing Amyloodinium ocellatum trophont (Arrowheads) and its attachment site to the destructed secondary gill lamella (Arrow) H&E X400 |
|
Figure 25 Showing Amyloodinium ocellatum trophont (Arrowhead) surrounded with tissue debri underneath a scale; Inset: high power of the A. ocellatumtrophont H&E X100, (inset, X1000) |
|
Figure 26 Showing Amyloodinium ocellatum trophont (Arrowheads) attached to the skin causing hyperplasia and desquamation of the covering epithelium (Arrows) H&E X400 |
|
Figure 27 Showing Amyloodinium ocellatum trophont (Arrowheads) and its attachment site to the destructed secondary gill lamella (Arrow) H&E X400 |
2.5 Treatment trials for Amyloodiniosis Results
2.5.1 Clinical signs before and after treatment
Before treatment the infected fish showed signs of distress loss of appetite flashing behavior mass mortality accumulation at water surface. Microscopic examination of skin and gill filament showed presence of tomont stage dark-brown in color mechanically dislodged between gill filaments.
After treatment feed consumption returned to a normal rate fishes in two treated groups resumed feeding from second day of commencement of treatment. Fish stopped dying increase vitality and trophont count decreased significally following treatment.
Effects of treatments on detachment of the trophonts and recovery of fish were studied the detachment of trophonts and its numbers were assessed by examining the gill filament skin and fin swab from the fish after treatment as in Table 5. The final mean of mortality percentage in each treated group was calculated on termination of treatment as in Table 4.
|
Table 4 Showing the final mean of mortality percentage in each treated group that was calculated on termination of treatment Note: Percents within the same column sharing the same subscript are not significantly different at p< 0.05 in the two-sided test Z-test. Tests are adjusted for all pairwise comparisons using the Bonferroni correction |
|
Table 5 Showing effects of treatments on detachment of the trophonts and recovery of fish |
As shown in Table 4 and Table 5, the mortality rate was decreased in the treated groups G1 and G2 to become none in the second week. Also, the intensity of infection was decreased in the treated groups where skin smear and gill smear shown decreased no of Amyloodinium than the non-treated group. Therefore, the prevalence rate was lower in the treated groups than non-treated. It was cleared that, this treatment with was more effective and excellent. It was confirmed through lower mortality rate and 0 percent prevalence rate.
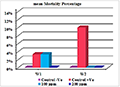
According to Table 6 and Figure 28, there was no significance difference in mean mortality percentage in treated group with 100 ppm and infested group in 1st week & 2nd week. Also there was no significance difference in mean mortality percentage between treated groups 100 ppm & 200 ppm. (1% & 0%) respectively in 1st week and in 2nd week (0% & 0%) respectively.
|
Table 6 Comparison of Mortality number and percentage among treated groups No. (%) after (30 min.) treatment (.G: group W week) Note: Percents within the same column sharing the same subscript are not significantly different at p< 0.05 in the two-sided test Z-test. Tests are adjusted for all pairwise comparisons using the Bonferroni correction |
|
Figure 28 Comparison of the mean Mortality number No. and percentage (%) among treated groups after (30 min.) treatment |
Table 7 and Figure 29 revealed that there was a significant difference in skin smear parasitic count between the infested groups (G2) that used as a control in the treatment and treated groups (G3 & G4). Skin smear parasitic count was recorded 12in 1st week while 10 in 2nd week. while in treated groups G3 with adose 100 ppm the skin smear parasitic count was recorded 6in 1st week & 3in the 2nd week and about the treated groups G4 with adose 200ppm the skin smear parasitic count was recorded 3in 1st week while 2 in 2nd week. It was cleared that there was no a major significant difference in prevalence rate percentage between the two doses that used in treatments design in 1st week & 2nd week.
|
Table 7 Comparison of skin smear parasitic count among treated groups after (30 min.) treatment (G: group; W: week) Note: Means within the same column carrying different superscripts are sig. different at P < 0.05 based on Tukey's Honestly Significant Difference (Tukey’s HSD) |
|
Figure 29 Comparison of skin smear parasitic count among treated groups after (30 min.) treatment |
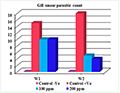
Table 7, Tabe 8, Figure 29 and Figure 30 were concluded that the mean intensity of infection (skin smear parasitic count & gill smear parasitic count that the load of infection in gill smear was higher than in skin smear (18&10) respectively. That means that the primary site of infection and the main target organ of amyloodinium ocellatum trophont is the gill tissues.
|
Table 8 Comparison of gill smear parasitic count among treated groups after (30 min.) treatment (G: group; W: week) Note: Means within the same column carrying different superscripts are sig. different at P < 0.05 based on Tukey's Honestly Significant Difference (Tukey’s HSD) |
|
Figure 30 Comparison of gill smear parasitic count among treated groups after (30 min.) treatment |
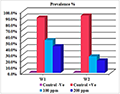
Table 9 and Figure 31 revealed that there was a significant difference in prevalence rate percentage between the infested groups (G2) that was recorded with a high prevalence % (90.0% in 1st week &93.3% in 2nd week) respectively, while the treated groups (G3) with adose100 ppm recorded (53.3% in 1st week &26.7% in 2nd week) respectively, the treated groups (G4) with adose 200 ppm recorded 43.3% in 1st week &20.0% in 2nd week) respectively, also it was cleared that there was no a major significant difference in prevalence rate percentage between the two doses that used in treatments design, in 1st week & 2nd week.
|
Table 9 Comparison of prevalence % among treated groups after (30 min.) treatment (G: group; W: week) Note: Percents within the same column not sharing the same subscript are significantly different at p< 0.05 in the two-sided test Z-test. Tests are adjusted for all pairwise comparisons using the Bonferroni correction |
|
Figure 31 Comparison of prevalence % among treated groups after (30 min.) treatment |
The infested fishes were exposed to 100 ppm & 200 ppm hydrogen peroxide 20% for thirty minutes. The control fish were examined and found to have a mean of 18 ± 0.93 trophonts per gill biopsy & 10 ± 0.91 trophonts per skin smear. The fish that were to be treated with100ppm showed a mean of 10 ± 1.01 trophonts per gill biopsy in first week & 6 ± 1.11 trophonts per skin smear. The fish were retreated with 100 ppm hydrogen peroxide for thirty minute for another 6 days and the count was down to 5 ± 0.93 trophonts per gill biopsy & 3 ± 0.83 trophonts per skin smear. The fish were treated with another dose 200 ppm hydrogen peroxide for thirty minute for 6 days. the fish that were to be treated with 200 ppm showed a mean of 10 ± 0.93 trophonts per gill biopsy in first week & 3 ± 1.03 trophonts per skin smear. The fish were retreated with 200 ppm hydrogen peroxide for thirty minute for another 6 days; the count was down to 4 ± 0.93 trophonts per gill biopsy & 2 ± 0.97 trophonts per skin smear.
3 Discussion
Eissa (2002) reported that, by increasing intensification of fish production and lack of health management measures have led to many disease problems. About 80% of fish disease is parasitic especially in warm water fish. The ectoparasitic protozoal diseases (gill and skin parasites) of fishes play an effective role in the economic losses of fish farms through mortality and/or decrease growth rate of fish especially in the highly intensified systems.
Pereira et al. (2010) reported that among the most important ectoparasitic protozoa is Amyloodinium ocellatum, a dinoflagellate which causes one of the most serious diseases of warmwater marine aquaculture and the disease caused by this organism is commonly referred to as amyloodiniosis or marine velvet disease. Lom and Dikov (1992) reported changes in fish behavior, with jerky movements, swimming at the water surface and decreased appetite. Levy et al. (2007) suggested that, the first indication of an amyloodinium infection is dead or dying fish. Behavioral signs may include a decrease in or complete lack of feeding activity, flashing (rubbing against objects in the tank or on the bottom substrate) and coughing (back flushing water across the gills). The skin of heavily infected fish may have a dull gold or brown sheen. And also may reveal scale loss and patchy accumulation of mucus. Levy et al. (2007) observed that, infected fish sometimes develop a white or brown coloration (“velvet”) or cloudy appearance, If the primary site of infection is skin, which is most visible when viewed with indirect lighting such as a flashlight. Such fish may display signs of “flashing” or rubbing on tank walls, the substrate, or other structures in their environment. Again, feeding behavior likely will be poor and some fish may appear emaciated. Fish with Amyloodinium infection alone do not typically have ulcers, white spots, or fuzzy lesions, but the skin can seem “hazy” in appearance. If the infection is confined to the gill, the “velvet” appearance will not be present.
The present study showed that infested European seabass Dicentrarchuslabrax with Amyloodinium ocellatum appeared distressed, emaciated, and anorexic and showed flashing behavior. Also, there were gasping of air rapid gill movement and lying on the bottom. There was rapid and mass death of fish. The affected skin showed friable skin (velvet like appearance), darkened, excessive mucous secretions forming cloudy film of slime. Also, grossly focal erosion areas were seen on the operculum and caudal tail. Schwarz and Smith (2009) observed by Light microscopy of moribund and dead fry showed brownish or yellowish, round to oval-shaped trophonts attached to the body of the fish. The infestation on July resulted to a sudden mass mortality. Some trophonts might have completed its feeding stage and detached to become a tomont. Lom and Dikov (1992) mentioned that the method of diagnostic is the identification of the trophont in the skin and gills of infected fish, which can be done through microscope observation of these tissues. Trophonts are ovoid with 150-350 microns. Gills are first to be infected.
In this study it was concluded that intensity of infection in gill tissues was higher than in skin tissues respectively. That indicates that primary site of infection and the main target organ of is gill tissues. Also that be confirmed from the histopathologcal diagnosis that revealed mild hyprer plasia in skin tissue while in gill tissue was sever. In this study Microscopic examination of skin and gills scrapings of examined D. labrax showed, round to oval small dark brown mucoid Amyloodinium ocellatum stage. Different developmental life stages of Amyloodinium ocellatum were seen in the gill and skin tissues as the two main inhabitant organs. The first stage was trophonts with its root - like structure (Rhizoids). They showed distended appearance with the presence of feeding stage trophonts dark brown color lodged between the gill filaments. Also, dislodged trophonts were also observed between the skin and fin surface. In detached trophonts (feeding stage) the rhizoids root-like structure (that penetrates deep in to epithelium causing substantial damage to tissue at the attachment site)of the attached organelle was sometimes visible. The second was tomont stage; trophont stage feeds for several days, detaches, retracts its rhizoids and becomes tomont. Tomonts (reproductive stage) in division were occasionally observed. The third was Dinospores, the tomont divides, producing Dinospores, were not observed as it is a free swimming stage in water. Paperna (1980) and Johnson (1990) mentioned that histologically, affected gills may appear hyperplastic (a proliferation of cells) and lamellar fusion may be evident. Paperna (1980) suggested that A. ocellatum is attached to and feeds from the host epithelial cell by means of rhizoids, which penetrate the host cell. The consumed cell gradually degenerates and collapses.
Damage to infected cells leads to focal erosion of the epithelium. Prolonged infection exhausts a generation of mucus cells and leads to accelerated desquamation. Proliferation of the epithelium causes obliteration of the gill lamellae, while the inner strata of the epithelium become spongious and in some cases undergo complete lysis. The histopathological diagnosis in present study revealed mild to severe histopathological changes. Severe hyperplasia was shown in the gill epithelia and fusion of the secondary gill lamella which was caused by Amyloodinium ocellatum trophonts. Also Destructed secondary gill lamellawhich was caused by Amyloodinium ocellatum trophont attachment to gill tissue was observed. Amyloodinium ocellatum trophont was surrounded with tissue debri underneath a scale. Mild Hyperplasia and desquamation of the covering epithelium which was caused byattachment of Amyloodinium ocellatum trophont to the skin tissue. Environmental and seasonal dynamics of A.ocellatum infestation is documented by Kuperman and Matey (1999) and Pereira et al. (2010). Noga and Levy (1995) reported that Infestations of the gilthead seabream by the gill parasite A. ocellatum may occur, particularly during summer. At the end of the growing season, when the water temperature increases within production ponds, the proliferation of the ectoparasite and the damage done to the developing fish can rapidly reach devastating proportions in terms of fish production. In Portugal, Menezes (1994) reported several outbreaks of A. ocellatum in aquacultures from the central west, southwest and southern regions where the main fish species in production are gilthead seabream and seabass (Dicentracthus labrax L.). Fish are usually introduced into production ponds in April, and captured in the following year, during summer. The growing period of gilthead seabream takes about 15 months. Infestations of the gilthead seabream by the gill parasite A. ocellatum may occur, particularly during summer. Noga and Levy (1995) suggested that at the end of thegrowing season, when the water temperature increases within production ponds, the proliferation of the ectoparasite and the damage done to the developing fish can rapidly reach devastating proportions in terms of fish production. Low oxygen tension in the Salton Sea in the summer months may reinforce the negative impact of Amyloodinium ocellatum. The shortage of external oxygen, together with destructive alterations of the respiratory organs and distortion of epithelia1 tissues caused by parasitic trophonts may depress the respiratory functions of fish. The likelihood of death by suffocation is especially great for young fish heavily infected by parasitic trophonts. In this case, not only gas exchange in the gills but also cutaneous respiration as a main source of oxygen for these fish (Rombough and Ure, 1990) may have been reduced. Alterations in the water-salt balance processes in the damaged gills were also suspected to occur (Bonga, 1997). The developing immune system of such young fish may not be able to fight off infection successfully. The present study to explain the occurrences per season of A. ocellatum in cultivated European seabass. Out of the examined 1065 Dicentrarchuslabrax fish, 618 (58.02%) were found to be infested with Amyloodinium ocellatum. The highest rate of infestation was recorded during spring season with aprevalence (90.10%) while the lowest was during winter season with aprevalence (30.76%). Khan and Thulin (1991) mentioned that, environmental factors can strongly promote infestation of fish by external parasites In the Salton Sea, A. ocellatum infestation of young tilapias increased under unfavorable environmental conditions of the lake.
The severity of fish infestation by A. ocellatum was determined by an interaction by te pathogen with abiotic variables, such as water temperature, salinity, oxygen concentration and nitrogen level. Kuperman and Matey (1999) suggested that the occurrence of A. ocellatum in fish gills is associated with specific variances in environmental variables such as temperature and salinity. A correlation study with environmental and biological factors as variables the occurrences of A. ocellatum in cultivated gilthead seabream) is conducted by Pereira et al. (2010). The study concludes that salinity is positively related with trophont occurrences. Dissolved oxygen, water temperature, pH, and phytoplankton biomass have significant negative relationship with A. ocellatum trophonts. Pereira et al. (2010) is conducted the correlation study with environmental and biological factors as variables to explain the occurrences of A. ocellatum in cultivated gilthead seabream.
Salinity is positively related with trophont occurrences. Dissolved oxygen, water temperature and pH, have significant negative relationship with A. ocellatum trophonts. California Regional Water Quality Control Board (1994) found that, the parasitic dinoflagellate Amyloodinium ocellatum appears to be as an important factor affecting survival of fish populations in the Salton Sea. The parasite infestation of young tilapia increased under unfavorable conditions at the lake. The massive fish mortality events often reported at the Salton Sea may be the result of synergistic effects of parasite load and a complex set of environmental stressors. Salinity is the only environmental variable positively correlated with A. ocellatum trophonts, having an important role in parasite occurrences that is also supported, in part, by Kuperman and Matey (1999), who pointed out that a combination of high water temperature and high salinity levels promoted heavy infestation by A. ocellatum. Thus, the present study attempts to correlate environmental factors with A. ocellatum, occurrences in cultivated European seabass. The present study concluded that there was direct strong significant correlation between pH levels and parasitic infestation. Also, there was astrong significant correlation between Salinity levels and parasitic infestation. There was a weak significant correlation between ammonia levels and parasitic infestation. Also, there was a significant correlation between water temperature levels and parasitic infestation while there was no significant correlation between dissolved oxygen and A. ocellatum Occurrences. Montgomery-Brock et al. (2001) reported that, Treatment with hydrogen peroxide to 75-150 mg/L was effective in eliminating trophontes in Polydactylus sexfilis.
In the present study the infested fishes were exposed to 100 ppm & 200 ppm hydrogen peroxide 20% for thirty minutes. The control infested fishes were examined and found to have a mean of 18 ± 0.93 trophonts per gill biopsy & 10 ± 0.91 trophonts per skin smear. The fish that were to be treated with100ppm showed a mean of 10 ± 1.01 trophonts per gill biopsy in first week & 6 ± 1.11 trophonts per skin smear. While in after six days, the count was down to 5 ± 0.93 trophonts per gill biopsy & 3 ± 1.03 trophonts per skin smear .The fishes were retreated with 200 ppm hydrogen peroxide for thirty minute for another 6days. The fish that were to be treated with100ppm showed a mean of 10± 0.93trophonts per gill biopsy in first week & 3 ± 1.03 trophonts per skin smear. While in after six days, the count was down to 4 ± 0.93 trophonts per gill biopsy & 2 ± 0.97 trophonts per skin smear. Fishes that exposed to 100 ppm & 200 ppm of showed no mortalities recoreded.in the termination of experiment.
Acknowledgments
The authors would like to express their gratitude and appreciation to University of Mansoura in Egypt for giving them the opportunity to carry out this work, and I would like to especially thank to National Institute of Oceanography and Fisheries (N.I.O.F.) in Alexandria, for helping me during the measurements, useful comments and assistance.
Agius C., and Roberts R.J., 2003, Melanomacrophage centres and their role in fish pathology, J. Fish Dis., 26: 499-509
https://doi.org/10.1046/j.1365-2761.2003.00485.x
PMid:14575368
Alvarez-Pellitero P., 2008, Diseases caused by flagellates, IN: Fish Diseases, Volume 1, Eiras, J.C., H. Segner,T. Wahli, and B.G. Kapoor (eds), Science Publishers: Enfield, NH, pp.421-515
APHA, 2005, Standard Methods of Water and Wastewater, 21st Edn., American Public Health Association, Washington, D.C., ISBN: 0875530478, pp.2-61
Bonga S.E.W., 1997, The stress response in fish, Physiol Rev., 77:591 -625
California Regional Water Quallty Control Board, 1994, Water Quallty Control Plan Colorado River Basln-Region 7, State Water Resources Control Board
Eissa I.A.M, 2002, Parasitic fish diseases in Egypt, 1st. edition, pp.52-53
Johnson S.K., 1990, Recognition and control of diseases common to grow-out aquaculture of red drum. IN:Red Drum Aquaculture, G.W. Chamberlain, R.J. Miget, and M.G. Haby (eds), Texas A&M University Sea Grant College Program, College Station, TX. pp.113-130
Kuperman B.I., and Matey V.E., 1999, Massive infestation by Amyloodinium ocellatum (Dinoflagellida) of fish in a highly saline lake, Salton Sea, California, USA, Dis. Aquat. Org. 39: 65–73
https://doi.org/10.3354/dao039065
PMid:11407406
Khan R.A., and Thulin J., 1991, Influence of pollution on parasites of aquatic animals, In:Baker, J.R., Muller, R. (Eds.), Advances in parasitology, Vol. 30, Academic Press, London, pp.200–238
https://doi.org/10.1016/s0065-308x(08)60309-7
Kuhl H, Beck A, Wozniak G, Canario A.V.M., Volckaert F.A.M., and Reinhardt R., 2010, The European sea bass Dicentrarchus labrax genome puzzle: comparative BAC-mapping and low coverage shotgun sequencing, BMC Genomics, 11: 68–80
https://doi.org/10.1186/1471-2164-11-68
PMid:20105308 PMCid:PMC2837037
Levy M.G., Poore M.F., Colorni A., Noga E.J., Vandersea M.W., and Litaker R.W., 2007, A highly specificPCR assay for detecting the fish ectoparasite Amyloodiniumoscellatum, Diseases of Aquatic Organisms, 73: 219-226
https://doi.org/10.3354/dao073219
PMid:17330741
Lom J. and Dykova I., 1992, Protozoan parasites of fishes, In: Developments in Aquaculture and Fisheries Science, (Elsevier, Amsterdam), 26: 315
Menezes J., 1994, Doenças em peixes cultivados no Estuário do Sado e o seu controlo, Seminário sobre recursos haliêuticos, ambiente, aquacultura e qualidade do pescado da península de Setúbal, vol. 1. Instituto Português de Investigação Marítima, Lisboa, pp.175–186
Montgomery-Brock D., Sato V.T., Brock J.A., and Tamaru C.S., 2001, The Application of hydrogen peroxide as a treatment for the ectoparasite amyloodinium ocellatum (Brown 1931) on the Pacific Threadfin Polydactylus sexifilis" Journal of the World Aquaculture Society, Vol. 32, No. 2, pp.250-254
https://doi.org/10.1111/j.1749-7345.2001.tb01103.x
Noga E.J., 2010, Fish Disease: Diagnosis and Treatment, Second Edition, Wiley-Blackwell: Ames, IA. pp.13-48, 143-147, 375-420
https://doi.org/10.1002/9781118786758
Noga E.J., and Levy M.G., 2006, Phyllum Dinoflagellata, IN: Fish Diseases and Disorders, Volume I: Protozoan and Metazoan Infections, P.T.K. Woo (ed), CAB International: Oxford, UK, pp.16-45
Noga E.J., and Levy M.G., 1995, Dinoflagellate parasites of fish, In: Fish diseases I: Protozoan and metazoan infections, Vol. 1, Woo P.T.K. (ed), pp.1-25, CAB International, Oxfordshire, Wallington, United Kingdom, pp.808
Pereira J.C., Abrantes I., Martins I., Barata J., Frias P., and Pereira I., 2010, Ecological and morphological features of Amyloodinium ocellatum occurrences in cultivated gilthead seabream Sparus aurata L.: A case study. Aquaculture, 310: 289-297
https://doi.org/10.1016/j.aquaculture.2010.11.011
Paperna I., 1980, Amyloodinium ocellatum (Brown 1931) (Dinoflagellida) infestations in cultured marine fish at Eliat. Red Sea: epizootiology and pathology. J. Fish Dis., 3: 363–372
https://doi.org/10.1111/j.1365-2761.1980.tb00421.x
Rombough P.J., and Ure D., 1990, Partioning of oxygen uptake between cutaneous and branchial surfaces in larval and juvenile chinook salmon, Onchorchynchus tschacvytscha. Physiol Zoo1, 64(7): 17-72
Schwarz M.H., and Smith S.A., 2009, Getting acquainted with Amyloodinium ocellatum, Commercial Fish and Shellfish Technology, Virginia Cooperative Extension. Fact Sheet, Pub. 600-200, Virgina, USA, pp.2
Saraiva A., Jerónimo D., and Cruz C., 2011, Amyloodinium ocellatum (Chromalveolata: Dinoflagellata) in farmed turbot, Aquaculture, 320: 34 -36
https://doi.org/10.1016/j.aquaculture.2011.07.034
Zorrilla I., Chabrillón M., Arijo S., Díaz-Rosales P., Martínez - Manzanares E., Balebona M.C., and Moriñigo M.A., 2003a, Bacteriarecovered from diseased cultured gilthead sea bream(Sparus aurata L.) in southwestern Spain. Aquaculture, 218: 11–20
. PDF(1324KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Samar Saad Mohamed Seoud
. Viola H. Zaki
. Gamal E. Ahmed
. Nevien K. Abd El-Khalek
Related articles
. Amyloodinium ocellatum
. European seabass fishes
. Infestation
. Treatment
. Dicentrarchus labrax L.
. Egypt
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)

.png)
.png)