Research Article
Estimation of Bacterial Biodegradability of PAH in Khor Al-Zubair Channel, Southern Iraq 
 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2017, Vol. 7, No. 42 doi: 10.5376/ijms.2017.07.0042
Received: 01 Sep., 2017 Accepted: 27 Sep., 2017 Published: 20 Oct., 2017
Alkanany F.N.A., Gmais S.A., Maki A.A., and Altaee A.M.R., 2017, Estimation of bacterial biodegradability of PAH in Khor Al-Zubair channel, Southern Iraq, International Journal of Marine Science, 7(42): 399-410 (doi: 10.5376/ijms.2017.07.0042)
Four bacterial strains were isolated from Khor Al-Zubair channel, southern Iraq based on a high growth rate on crude oil and on hydrocarbon degradation ability. The strains were preliminarily identified based on morphological observation, and by the Vitek II system. The isolates were identified as Brevundimonas diminuta /vesicularis, Vibrio vulnificus, Sphingomonas paucimobilis and Ochrobactrum anthropic. However, the ability of these strains to utilize crude oil (0.25%; 0.5%; 1% and 2% v/v) varied both in rates of utilization and in growth profiles. The components of crude oil degraded by the isolates were determined by capillary gas chromatography. Sphingomonas paucimobilis and Vibrio vulnificus were the most effective bacteria degrade PAH on seventh day incubation, especially in concentration 2.0 mg/l (97.39% and 84.23%) respectively, meanwhile, Brevundimonas diminuta/ vesicularis was highly effective degrade PAH (73.41% and 62.08%) in concentration 1.0 and 0.5 mg/l, respectively. On the other hand, Ochrobactrum anthropi was the lowest in all concentrations.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are compounds consisting of two or more aromatic rings, the USEPA has reported sixteen unsubstituted PAHs as main concern pollutants, eight of them may be human carcinogens (Yan et al., 2004). The concern is that some PAHs are toxic, carcinogenic or teratogenic. PAHs are a resource of carbon and energy and being exploited by some bacteria as growth substrates. The presence of PAH in the marine environment is largely attributed to oil spills, discharge and natural river infiltration, import or transfer of airflow. Therefore, global increased human activity has increased risks to the marine environment (Latimer and Zheng, 2003).
PAHs are present as small and large, depending on the number of rings. "Small" PAHs contain up to six condensed aromatic rings, whereas, "large" PAHs containing more than six aromatic rings, which are less soluble in water and less volatile (Kumar et al., 2011).
Although PAHs fact that undergo oxidation, photolysis, bioaccumulation, volatilization and adsorption in the environment, but have been microbial degradation and transformation as the key processes identified for the removal of pollutants (Zeng et al., 2010).
Biodegradation is a viable bioremediation technology for organic pollutants. It has identified that microorganism degrade ecological pollutant in various environments. Bioremediation uses the metabolic adaptation of microorganism to degrade contaminations. Bioremediation is a promising approach, as it employs microorganisms to degrade or remove different pollutants, such as organic compounds into harmless metabolites or mineralize the contaminants to CO2 and water (Alexander et al., 1985).
The rate of biodegradation depends on pH, temperature, microbial population, oxygen, nutrient accessibility, chemical structure of the compound, the division of properties and cellular chemical transport in the growth medium (Singh and Ward, 2004). The aim of the present study was to isolate and identify new bacteria that have the ability to degrade polycyclic aromatic hydrocarbons.
1 Materials and Methods
1.1 Collection of samples
Samples were collected from Khor Al-Zubair channel, southern Iraq (Figure 1) from five stations. Water samples (500 ml) were collected at two depths in sterile glass bottle, all samples transferred to an ice box and transported to the laboratory.
|
Figure 1 Sampling locations |
1.2 Isolation and identification of bacteria
One milliliter of each sample was cultured in a conical flask containing 100 ml mineral salts medium (MSM), the composition of the MSM was 0.3 gm of KCl, 1.0 gm of K2HPO4, 0.5 gm of KH2PO4, 0.01 gm of FeSO4.7H2O, 30.0 gm of NaCl, 0.5 gm of MnSO4.7H2O, 0.2 gm of CaCl2 and 1000 ml DW (Fujisawa and Murakami, 1980). Four concentrations of crude oil as 0.25 ml, 0.5 ml, 1 ml and 2.0 ml (Provided by Al-Shua’aba Refinery) were added separately to the medium. Decimal dilutions of 7 days grown culture was cultivated at 30°C.
Nutrient agar (Hi media- India) with 30 ppt sodium chloride and marine agar (Difco, USA) were used to isolate Vibrio vulnificus, Brevundimonas diminuta/ vesicularis, Sphingomonas paucimobilis and Ochrobactrum anthropic and identified by the Vitek II system (VK2C8300, USA).
1.3 Degradation of PAH
One millilitre of broth culture of each bacterial isolate was incubated separately in 250 ml Erlenmeyer flask containing 50 ml of MSM at 20°C for 7 days at 120 rpm using a cooling incubator shaker (Al-Sulami et al., 2014). All the experiments were carried out in two duplicates, and the residual crude oil was performed after 7 days.
1.4 Gas chromatographic analysis of residual crude oil
The residual crude oil was extracted by liquid-liquid extraction, as described by Adebusoye et al. (2007). The aqueous phase was removed by separating funnel and the residual oil dried in the oven at 40°C to remove CCl4.
The aromatic fraction was separated using separation column (25 cm length, 3 cm diameter) containing 8 gm of silica gel over a little amount of glass cotton (Farid, 2006). The residual oil dissolved in 25 ml of benzene and poured into the separation column and drawn off the aromatic fraction in 50 ml beaker. Control flasks were also extracted similarly, aromatic fraction hydrocarbons estimated by FID gas chromatography technique (Agilent Chem Station).
2 Results and Discussion
2.1 Identification of bacteria
Sixty five isolates of gram negative bacilli was isolated from Khor Al-Zubair channel southern Iraq, Fifty-six of them were identified correctly to the species level as V. vulnificus, Sphingomonas aucimobilis, Brevundimonas diminuta/ vesicularis and Ochrobactrum anthropi, while 9 strains were not identified. Direct identification, reporting time of the VITEK II was 4.5 to 10 hours after incubation (Table 1). Four species were isolated by the VITEK II system and their ability to degrade four concentrations of crude oil was examined.
|
Table 1 Identification of bacteria isolates by Vitek II |
2.2 Degradation study of PAH
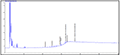
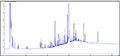
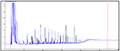
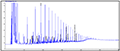
In the present study, four species were examined their capability to degrade 4 concentrations of crude oil. Table 2 shows the percentage of degradation of PAH for each genus. Sphingomonas paucimobilis was the most effective bacteria to degrade PAH (97.39%) especially in concentration 2.0 mg/l, while Ochrobactrum anthropi was the lowest (13.15%) in the same concentration. Figure 2, Figure 3, Figure 4 and Figure 5 show GC results of control samples (0.25%; 0.5%; 1% and 2%). Brevundimonas diminuta/ vesicularis showed high PAH degradation (73.41%) in concentration 1.0 mg/l and low degradation (25.66%) in concentration 2.0 mg/l (Table 2; Figure 6; Figure 7; Figure 8; Figure 9).
|
Table 2 Degradation percentage of PAH |
|
Figure 2 Crude oil (aromatic faction 0.25%) |
|
Figure 3 Crude oil (aromatic fraction 0.5%) |
|
Figure 4 Crude oil (aromatic fraction 1.0%) |
|
Figure 5 Crude oil (aromatic fraction 2.0%) |
|
Figure 6 Crude oil 0.25% with Brev. dimi./vesicu. |
|
Figure 7 Crude oil 0.5% with Brev. dimi./vesicu. |
|
Figure 8 Crude oil 1.0% with Brev. dimi./vesicu. |
|
Figure 9 Crude oil 2.0% with Brev. dimi./vesicu. |
On the other hand, Vibrio vulnificus showed high degradation percentage of PAH (84.23%) in concentration 2.0 mg/l and low degradation (22.91%) in concentration 0.5 mg/l (Table 2; Figure 10; Figure 11; Figure 12; Figure 13). The genus of Vibrio was first described as a degrading phenanthene organism from West and colleagues, who isolated large numbers of strains by spreading Chesapeake Bay samples onto complex media each with a superficial layer of phenanthrene (Okpokwasili et al., 1984; West et al., 1984).
|
Figure 10 Crude oil 0.25% with V. vulnificus |
|
Figure 11 Crude oil 0.5% with V. vulnificus |
|
Figure 12 Crude oil 1.0% with V. vulnificus |
|
Figure 13 Crude oil 2.0% with V. vulnificus |
Sphingomona paucimobilis showed high degradation percentage (97.39%) in concentration 2.0 mg/l and low degradation (27.26%) in concentration 0. 5 mg/l (Table 2; Figure 14; Figure 15; Figure 16; Figure 17). This is consistent with a report by Tao et al. (2007) who found that this bacterium has unique genes to degrade a wide range of PAH and related compounds. The gene is related from other Pseudomonas genera and reports so far in sequence homology and gene organization. Also Pinyakong et al. (2003a; 2003b) observed that, many sphingomonads degrade naphthalene, anthracene, and phenanthalene by common pathways were found in other gram-negative bacteria. In addition, many researchers (Kim et al., 1996; Feng et al., 1997; Romine et al., 1999; Ogram et al., 2000; Basta et al., 2004) found large plasmids in strains of Sphingomonas xenobiotic contributing to the degradation of PAH.
|
Figure 14 Crude oil 0.25% with Sphingomon. Paucimobilis |
|
Figure 15 Crude oil 0.5% with Sphingomon. paucimobilis |
|
Figure 16 Crude oil 1.0% with Sphingomon. paucimobilis |
|
Figure 17 Crude oil 2.0% with Sphingomon. paucimobilis |
Pure and mixed cultures of bacteria isolated from fresh water and seawater have the capacity to metabolize, anthracene, phenanthrene and as the only source of carbon anthracene are completely mineralized. Pseudomonas, Sphingomonas, Nocardia, Beijerinckia, Rhodococcus and Mycobacterium with the initial oxygenated intermediate being a dihydriol (Mrozik et al., 2003).
While Ochrobactrum anthropic showed low degradation percentage of all concentrations (Table 2; Figure 18; Figure 19; Figure 20; Figure 21). Sulaiman et al. (2016) found that, out of 35 isolates obtained from petroleum- contaminated soil only five isolates have the ability to degrade phenanthrene and anthracene. Whereas Katsivela et al. (2002) found that, 97% of 2; 2; 4; 4; 6; 8; 8- heptamethylnonane, 55% of toluene, 71% of acenaphthylene and 72% of acenaphthene were depleted after 9 days of growth. The ability of these bacteria to remove different PAHs looks promising for use in bioremediation technologies.
|
Figure 18 Crude oil 0.25% with Ochrobac. anthropi |
|
Figure 19 Crude oil 0.5% with Ochrobac. anthropi |
|
Figure 20 Crude oil 1.0% with Ochrobac. anthropi |
|
Figure 21 Crude oil 2.0% with Ochrobac. anthropi |
Biodegradation of PAHs as described by Johnsen et al. (2005) can serve three different functions:
1- Assimilative biodegradation that yields carbon and energy for the microorganism and goes along with the mineralization of the compound.
2- Intracellular mechanism of PAHs is soluble in water and therefore causes excretion.
3- Cometabolism which is the degradation of PAHs without production of energy and carbon for the organism metabolism.
These differences between the bacterial degradation of only the PAH environment probably reflect the diversity in the catabolic systems PAH and general physiology and possibly mediated ecological differences that are not understood (Brian et al., 2001).
The degradation of aliphatic hydrocarbons and PAH was mainly of the mono- and dioxygenase produced by bacteria. Therefore, the presence of six key enzymes coding genes, including both monooxygenase and dioxygenase in the ASU-06 strain of S. koreensis the existence of monooxygenase genes (alkB and alkB1) dioxygenase (naháč) and Catechol dioxygenase (C12O and C23O) genes was confirmed. Therefore, one possible reason may be that these genes are strong evidence between different conserved Gram-negative bacteria (Hesham et al., 2014).
3 Conclusion
In this study, 4 oil degrading bacterial strains were isolated from brackish water. The bacteria were identified as Brevundimonas diminuta/ vesicularis, Vibrio vulnificus, Sphingomonas paucimobilis and Ochrobactrum anthropic based on morphological properties and by Vitek II system. We found a variation in their capabilities to degrade PAH and most effective in higher concentrations. Perhaps, this is related to the types of bacteria and the mechanisms which to degrade crude oil.
Authors’ contributions
AMR designed and conducted research, and also arranged all things to complete this paper. Co-authors are contributed to collection samples from Khor Al-Zubair channel, southern Iraq and analyze the samples.
Acknowledgment
The authors would like to express their gratitude to the microbiology laboratory, Al- Sader Hospital, Missan, Iraq.
Adebusoye S.A., Ilori M.O., Amund O.O., Teniola O.D., and Olatope S.O., 2007, Microbial degradation of petroleum hydrocarbons in a polluted tropical stream, World J. Microbiol. Biotechnol., 23:1149-1159
https://doi.org/10.1007/s11274-007-9345-3
Alexander R., Kagi R.I., Rowland S.J., Sheppard P.N., and Chirila T.V., 1985, The effect of thermal maturity on distribution of dimethyl naphthalenes and trimethyl naphthalenes in some ancient sediments and petroleums, Geochim. Cosmochim. Acta., 49: 385-395
https://doi.org/10.1016/0016-7037(85)90031-6
Al-Sulami Amin A., Altaee Asaad M.R., and Al-Kanany Faduil N.A., 2014, Improving oil biodegradability of ailiphatic crude oil fraction by bacteria from oil polluted water, Afr. J. Biotechnol., 13(11): 1243-1249
https://doi.org/10.5897/AJB2013.13151
Basta T., Keck A., Klein J., and Stolz A., 2004, Detection and characterization of conjugative degradative plasmids in xenobiotics degrading Sphingomonas strains, J. Bacteriol., 186: 3862–3872
https://doi.org/10.1128/JB.186.12.3862-3872.2004
Brian P. Hedlund and James T. Staley, 2001, Vibrio cyclotrophicus sp. nov., a polycyclic aromatic hydrocarbon (PAH)-degrading marine bacterium, Internat. J. System. Evolut. Microbiol., 51: 61–66
https://doi.org/10.1099/00207713-51-1-61
Farid W.A.A., 2006, Effect of plant extracts on the microbial degradation of petroleum, Thesis of Phd. University of Basrah, Iraq
Feng X., and Ogram A., 1997, Plasmid-mediated mineralization of carbofuran by Sphingomonas sp. CF-06, Appl. Environ. Microbiol., 63: 1332–1337
Fujisawa H., and Murakami M., 1980, Method for screening–oxidizing bacteria in the sea, j. Shimonosekiuviv fish, 28: 101-108
Hesham A.E., Mawad A.M.M., Mostafa Y.M., and Shoreit A., 2014, Biodegradation ability and catabolic genes of petroleum-degrading Sphingomonas koreensis strain ASU-06 isolated from Egyptian oily soil, BioMed. Res. Intern., p.10
Johnsen A.R., Wick L.Y., Harms H., 2005, Principles of microbial PAH- degradation in soil, Environ. Pollut., 133: 71-84
https://doi.org/10.1016/j.envpol.2004.04.015
Katsivela E., Moore E., and Kalogerakis N., 2002, Biodegradation of aliphatic and aromatic hydrocarbons: specificity among bacteria isolated from refinery waste sludge, Water, Air, and Soil Pollution Focus, 3: 103–115
https://doi.org/10.1023/A:1023938003361
Kim E., Aversano P.J., Romine M.F., Schneider R.P., and Zylstra G.J., 1996, Homology between genes for aromatic hydrocarbon degradation in surface and deep- subsurface Sphingomonas strains, Appl. Microbiol. Biotechnol., 62: 1467–1470
Kumar A., Munjal A., and Sawhney R., 2011, Crude oil PAH constitution, degradation pathway and associated bioremediation microflora: an overview, Internat. J. Environ. Sci., 1(7): 1427-1446
Latimer J.S., and Zheng J., 2003, The sources, transport, and fate of PAHs in the marine environment, in PAHs: an ecotoxicological perspective, John Wiley & Sons, Ltd, UK, 7–33
https://doi.org/10.1002/0470867132.ch2
Mrozik A., Piotrowska-Seget Z., and Labuzek S., 2003, Bacterial degradation and bioremediation of polycyclic aromatic hydrocarbons, Polish J. Environ. Stud., 12 (1):15-25
Ogram A.V., Duan Y.P., Trabue S.L., Feng X., Castro H., and Ou L.T., 2000, Carbofuran degradation mediated by three related plasmid systems, FEMS Microbiol. Ecol., 32: 197–203
https://doi.org/10.1111/j.1574-6941.2000.tb00712.x
Okpokwasili G.C., Somerville C.C., Grimes D.J., and Colwell R.R., 1984, Plasmid-associated phenanthrene degradation by Chesapeake Bay sediment bacteria, Colloq Inst Francaise Rech Exploit Mer., 3: 601–610
Pinyakong O., Habe H., and Omori T., 2003a, The unique aromatic catabolic genes in sphingomonads degrading polycyclic aromatic hydrocarbons, J. Gen. Appl. Microbiol., 49: 1–9
https://doi.org/10.2323/jgam.49.1
Pinyakong O., Habe H., Yoshida T., Nojiri H., and Omori T., 2003b, Identification of three novel salicylate 1-hydroxylases involved in the phenanthrene degradation of Sphingobium sp. strain P2, Biochem. Biophys. Res. Co., 301: 350–357
https://doi.org/10.1016/S0006-291X(02)03036-X
Romine M.F., Stillwell L.C., Wong K.K., Thurston S.J., Sisk E.C., Sensen C., Gaasterland T., Fredrickson J.K., and Saffer J.D., 1999, Complete sequence of a 184-kilobase catabolic plasmid from Sphingomonas aromaticivorans, F199. J. Bacteriol., 181:1585–1602
Singh A., and Ward O.P., 2004, Biodegradation and bioremediation series: soil biology, vol. 233, Springer-Verlag, New York
https://doi.org/10.1007/978-3-662-06066-7
Sulaiman A. Alrumman, Abd El-Latif Hesham, and Saad A. Alamri, 2016, Isolation, fingerprinting and genetic identification of indigenous PAHs degrading bacteria from oil polluted soils, J. Environ. Biol. 37: 75-81
Tao X.Q., Lu G.N., Dang Z., Yang C.Y., and Xiao Y., 2007, A phenanthrene-degrading strain Sphingomonas sp. GY2B isolated from contaminated soils, Process Biochemistry, 2007. 42(3): 401-408
https://doi.org/10.1016/j.procbio.2006.09.018
West P.A., Okpokwasili G.C., Brayton P.R., Grimes D.J., and Colwell R.R., 1984, Numerical taxonomy of phenanthrene degrading bacteria isolated from the Chesapeake Bay, Appl. Environ. Microbiol., 48: 988–993
Yan J., Wang L., Fu P.P., and Yu H., 2004, Photomutagenicity of 16 polycyclic aromatic hydrocarbons from the US EPA priority pollutant list, Mutat. Res., 557: 99-108
https://doi.org/10.1016/j.mrgentox.2003.10.004
Zeng J., Lin X., Zhang J., and Li X., 2010, Isolation of polycyclic aromatic hydrocarbons (PAHs): degrading Mycobacterium spp. and the degradation in soil, J. Hazard Mater. 183:718–723
. PDF(951KB)
. FPDF
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Fadhil N.A. Alkanany
. Satar A. Gmais
. Anwar A. Maki
. Asaad M.R. Altaee
Related articles
. Khor Al-Zubair
. Bacteria
. Vitek II system
. Gas chromatography
Tools
. Email to a friend
. Post a comment

.png)


.png)


.png)