Research Article
Contribution of Vegetation Structure on Carbon Assimilation Capacity of Mangrove Ecosystem: A Case Study from Negombo Estuary, Sri Lanka 
2 Department of Botany, The Open University of Sri Lanka, Nawala, Nugegoda, Sri Lanka
 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2017, Vol. 7, No. 46 doi: 10.5376/ijms.2017.07.0046
Received: 09 Nov., 2017 Accepted: 01 Dec., 2017 Published: 15 Dec., 2017
Umayangani M.A.D., and Perera K.A.R.S., 2017, Contribution of vegetation structure on carbon assimilation capacity of mangrove ecosystem: a case study from Negombo estuary, Sri Lanka, International Journal of Marine Science, 7(46): 439-446 (doi: 10.5376/ijms.2017.07.0046)
Mangrove ecosystems perform number of important ecological functions and provide a wide range of services at the local or national level and provide a unique combination of both organic matter production and sequestration, which is different from other coastal ecosystems. Mangroves are extensively used to extract twigs and branches for the construction of “Brush piles”, the predominant fishing devices in the Negombo estuary, Sri Lanka, believed to have one major impact on the vegetation structure followed by the changes in primary productivity and carbon assimilation capacity of the ecosystem. Present study was conducted with an objective of quantify and relationships between the vegetation structure and Gross Primary Productivity (GPP) of disturbed and natural mangrove stands in the Negombo estuary. Vegetation structural parameters were gathered according to the standard procedures and the measurement of Photosynthetically Active Radiation (PAR) used to calculate the Leaf Area Index (LAI) followed by the GPP. Highest values of vegetation structural parameters, LAI and GPP were recorded from estuarine waterfront and decreased with landwards, which indicated more active vegetation is near by the estuarine waterfront. Comparatively, vegetation structural/structural complexity (CI) and GPP/carbon assimilation capacity were superior in undisturbed/ natural mangrove stands (31.86-36.65 Mg ha-1y-1) than that was recorded in disturbed/ replanted mangrove areas (26.42-35.25 Mg ha-1y-1) at Negombo estuary. Statistically significant liner relationships (P<0.01) were revealed between vegetation structural complexity and GPP (CI=.0.498 GPP +28.208); structural complexity and Leaf Area Index (CI=0.0956 LAI +5.3551).
Background
Mangroves are a collection of trees, shrubs, palms or ground ferns which mainly flowering plants normally grow above mean sea level in the intertidal zone of the marine or estuarine environments in coastline, bays, estuaries, lagoons, backwaters and in the rivers, reaching upstream up to the point where the water still remains saline. These mangrove plants do not form a phylogenetically related group of species but are rather species from very diverse plant groups sharing common morphological and physiological adaptations to life in the intertidal zone, which have evolved independently through convergence rather than common descent (Kathiresan and Bingham, 2001; Mc Leod and Salm, 2006; Boullion et al., 2009).
Mangrove ecosystems perform many important ecological functions and provide a wide range of services at the local and national level. The importance of mangrove ecosystems as a resource derives both from the products obtained directly from the ecosystem and ecological services provided within and beyond its boundaries (Barbier, 2007; Ronnback et al., 2007). While providing the above benefits there are also threats faced by mangrove forests. These threats are mainly due to the anthropogenic activities.
The topography of the coastal areas, the low tidal amplitude, range 0.4 -0.6 m (Wijeratne, 2004), around Sri Lanka, restricts the intertidal zone, thus the mangroves are limited to a narrow belt along the sheltered coasts, especially around lagoons and estuaries. The major mangrove areas in Sri Lanka are located on the northern and eastern coasts. Recent investigations reveal that total extent of mangroves in Sri Lanka is 15670 ha (Forest Department, 2012).
While providing the above benefits there are also threats faced by mangrove forests. These threats are mainly due to the anthropogenic activities, such as extraction of twigs and branches for the construction of “Brush Piles”, reclamation of land for housing, agriculture and industrial purposes.
Negombo estuary is located in west coast and belongs to wet climatic zone of Sri Lanka. Total extent of estuary is approximately 3500 ha. A wet climate prevails in the area and the mean annual rainfall is approximately, 2025 mm. Being located in a populous coastal area, relatively a small extent of mangroves are observed to be natural and the others are man-made, cultivated by artisanal fishermen to obtain twigs and branches to construct “Brush Piles”, a traditional method adopted to catch fish in shallow estuarine waters used by some fisherman in Negombo estuary and contributes considerably to the subsistence economy associated with the estuary. Brush piles are constructed with twigs and branches obtained from the mangrove areas around the estuary.
It was recognized that the significance differences in vegetation structure of human affected mangrove areas and relatively undisturbed mangrove areas at the Negombo estuary. Hypothesized that changes in vegetation structure of the mangrove ecosystem effect on the primary productivity of the ecosystem.
1 Materials and Methods
1.1 Study area
Negombo estuary, located on the western coast of the Sri Lanka at 7.60° to 7.12° N, 79.40° to 79. 53° E. Based on the published reports and aerial photography, total extent of Negombo estuary (water surface) is 3502 ha. Distribution of mangroves around the estuary is limited due to low tidal amplitude followed by a narrow intertidal belt about 10 m wide. Floral diversity reported higher with fourteen (14) of true mangrove species and number of mangrove associate species reside in Negombo estuary (Pinto, 1982). Salinity of the estuary recorded between 1.08 and 15.23 mg/l and surface water temperature varies between 26.0°–34.1°C. The pH reported between 7.34–8.02 (Gammanpila et al., 2009). The annual rainfall is approximately, 2025 mm, whereas the maximum rainfall occurs during May to August (Van Zon and Benthem, 1994).
1.2 Sampling strategy
In order to gather data on mangrove vegetation structure and photosynthetically active radiation (PAR), 10 m wide belt transects were laid perpendicular to the estuarine shoreline at randomly selected places in disturbed and relatively undisturbed/natural mangrove areas in the Negombo estuary. Length of the transect was depend on the dense and heterogeneity of the mangroves. Each transect was divided in to 10 m x 10 m (100 m2) sub-plots and a total of forty (40) sampling plots were used for sampling in both disturbed and undisturbed/natural mangrove areas.
1.3 Vegetation structure
Standard methods were adopted to quantify the major structural variables of the mangroves stands, as described by Cintron and Novelli (1984), Kathiresan and Khan (2010). Structural variables i.e. diameter at breast height (dbh) and tree height of the mangrove stands were gathered from each study plot (100 m2) in the belt transects. Plants with less than 2.5 cm were excluded.
Complexity index (CI), was explained the overall estimate of the structural complexity of the vegetation (Holdridge et al., 1971; Kathiresan and Khan, 2010; Perera et al., 2013; Perera and Amarasinghe, 2016). CI was calculated using data on the number of species, stand density, basal area and height.
CI = Number of species x stand density x stand basal area x stand height x 10-5
1.4 Leaf area index (LAI)
Measurement of photosynthetically active radiation (PAR) absorption by the canopy used to estimate the leaf area index. The method described by Jayakody et al. (2008); Kathiresan and Khan (2010) has been used to calculate the leaf area index.
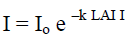
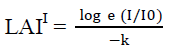
LAI = LAII x Cos (θ x 3.141593/180)
I = photon flux density beneath the canopy
Io = photon flux density incident on the top of the canopy or fully exposed position outside the canopy
LAII = Leaf area index correction
LAI = Leaf area index
θ = zenith angle of sun
k = canopy light extinction coefficient
A large number of measurements by Clough (1997); English et al. (1997) reported that k commonly lies between 0.40 and 0.65 in a variety of mangrove canopies and hence an average value of 0.5 was used for calculations. Light intensity of above canopy or in open space of the study area (I°) and under canopy (I) was recorded between 10.00 a.m. and 2.00 p.m. using LI-191SA line Quantum sensor.
Approximately 50 readings of I were taken from each study plot and accordingly 2000 readings (from 40 study plots) were obtained. Values of zenith angle (angle of the sun from the vertical) at the time that measurements of I were made, obtained from the Metrological Dept. of Sri Lanka and website www.solardat.uoregon.edu.
1.5 Gross primary productivity (GPP)
Gross primary productivity was estimated by using the Leaf area index (LAI) that was calculated using data on PAR absorption by the mangrove canopies. The potential gross primary productivity of the canopy per unit area was calculated with the following formula (Bunt et al., 1979; Clough, 1997).
GPP = A x d x LAI
Where d is the day length in hours, LAI is the leaf area index and A is the average rate of photosynthesis per unit area (g C m-2 hr-1) for all leaves in the canopy. This (value A) varied with climatic conditions. In harsh conditions (hot, dry and high soil salinity of 25 ppt), variable A is usually about 0.216 g C m-2 hr-1 and under favorable conditions (low salinity of less than 20 ppt), A may reach 1.0 g C m-2 hr-1 (Andrews and Muller, 1985; Clough and Sim, 1989; Cheeseman et al., 1997). While it is desirable to measure the actual rate of photosynthesis at each site and according to the explanation by English (1997), approximate rate of 0.216 g C m-2 hr-1 can be used for dry period, and 0.648 g C m-2 hr-1 for wet/raining conditions.
2 Results
2.1 Mangrove vegetation structure
|
Table 1 Summary of the vegetational structural parameters recorded in undisturbed and undisturbed mangrove areas at Negombo estuary |
As in many other mangroves ecosystems in Sri Lanka, Rhizophora mucronata, R. apiculata and Avicennia marina recorded the most dominant. In addition to that Lumnitzera racemosa, Excoecaria agallocha and Bruguiera spp are the major constituent species in mangrove areas of Negombo estuary. Relatively high stand density (5925 trees/ha) and high species composition were revealed in the mangrove ecosystem at Negombo estuary, where nine to ten true mangrove species were encountered in the subplots (4000 m2) used for the present study (Table 1). Relatively high vegetation structural complexity values were recorded close at the estuarine waterfront and decreases towards the landwards (Table 1).
2.2 Primary productivity and carbon assimilation capacity
|
Table 2 Leaf area index (LAI) and Gross primary productivity (GPP) values recorded in undisturbed and undisturbed mangrove areas at Negombo estuary |
2.3 Relationship between vegetation structure of mangroves and gross primary productivity (GPP)
|
Figure 1 Relationship between Leaf area index (LAI) and Vegetation structural complexity (CI) of mangroves |
|
Figure 2 Relationship between Gross primary productivity (GPP) and Vegetation structural complexity (CI) of mangroves |
3 Discussion
Mangrove ecosystems are among the most productive natural systems in the world. Besides productivity, several other functions and services, partly based on their high productivity, provide the surrounding human communities, and important resource based their livelihood. When considering the population distribution of Sri Lanka, high densities are reported along the west coast Negombo is one to the commercially important fishing centers in this coast, where density of population and population growth rate are high. Negombo estuary and mangrove communities subject to the present study are situated near this highly populated Negombo town area, especially in the northern end of the lagoon. Great pressure due to anthropogenic causes, therefore effect to a considerable extent, vegetation structure of mangroves. Despite these effects, Negombo estuary wet land system has serviced multiple users including fishing, agriculture, trade & fishing and habitation since a very long time.
According to the results obtained from the present study fishing is a major occupation in this area. Most of the human communities that associate the estuary are dependent on the direct utilization of common natural resources. Twigs or Brush Piles is the major use of mangrove resource base of the area and firewood, poles, posts & tannin are some of the forest products obtained from mangroves of Negombo estuary. Fishing techniques used by the lagoon fishermen are traditional and simple. Among the mangrove resource systems, Brush Piles fishery is the one that exclusively dependent on mangrove resource base. Currently the method of the Brush Piles technique is found to be the greatest harm to the mangrove vegetation. Further it may be causal in the degradation and change the structure of mangroves in Negombo estuary.
Density of trees and number of species are low in disturbed areas represented by lower complexity values, due to human interference. Areas where species of Rhizophora have been propagated by fisher folk were identified as cultivated mangrove stands at some islands.
Some areas in Negombo mangroves are planted by fishermen with many species of mangroves and they are categorized under managed areas, of which Wedikanda is the representative site. These mangrove areas are managed by the fishermen themselves. Structurally, managed areas are distinctly different from natural and mono-specific stands. In response to the scarcity of twigs and branches, fishermen plant mangroves of selected species. Species of Rhizophora are the most preferred. This appears as one of the main reasons for changing structure of the mangrove vegetation. The other reason for structural change is the over exploitation through cutting of mangrove branches.
The higher values of potential gross primary productivity (GPP) observed during and immediately after the raining season, at mangrove areas at Negombo estuary, may be due to the enhanced nutrient inputs from surface and river runoff and decreased soil salinity that in turn may lead to low leaf fall in comparison to dry period, thus resulting a higher leaf area index (LAI). The availability of freshwater indicated an important factor for development and growth of mangroves. Freshwater supply has often been indicated by the ratio of rainfall to evapotranspiration.
Rate of photosynthesis vary widely among the physical environmental factors as well as vegetation structural characteristics. Complexity index (CI) represents the vegetation structure of the mangrove stand, that contributes to functions of the mangrove ecosystem, revealed a strong positive relationship with the GPP calculated for Negombo estuary and similar relationships were reported by Jayakody et al. (2008) and Perera et al. (2010) for mangrove areas in Negombo estuary.
Woodroffe (1993) explain geomorphology and hydrology determined by local geology, sea-level change, tide, fresh water input, shoreline structure, watershed morphology, groundwater influence, natural disturbance regimes and climate, contribute to development of physio-chemical gradients which in turn govern the structure and function of the intertidal ecosystems. Many of the estuaries and lagoon in Sri Lanka can be categorized as riverine which receive continuous input of nutrient rich freshwaters through rivers and get mixed with saline waters resulting reduced salinity and this water inundates areas close to the shoreline most frequently than in the landward areas .High mangrove structural complexity, which is represented by high number of species, plant densities and heights, high leaf area indexes is observed with the mangrove areas close to the shoreline and it was revealed to decrease along the environmental gradient towards inland, thus GPP also declines along the water-land gradient in the estuary.
Authors’ contributions
Ms. M.A.D. Uumayangani contributed as a principal investigator of this research study and Dr. K.A.R.S. Perera contributed as a supervisor and advisor of this work.
Acknowledgments
Authors extend their gratitude to the facilities provided by the National Aquatic Recourses Research and Development Agency’s (NARA) Regional Centre at Kodalkele, Negombo, Sri Lanka, and generous assistant provided by Mr.W.A.Sumanadasa, at NARA, in collecting of field data.
Andrews T.J., and Muller G.J., 1985, Photosynthetic gas exchange of the mangrove, Rhizophora stylosa Griff in its natural environment, Oecologia, 65: 449–455
https://doi.org/10.1007/BF00378922
PMid:28310452
Barbier E.B., 2007, Valuing ecosystem services as productive inputs, Economic Policy Journal, 22: 177-229
https://doi.org/10.1111/j.1468-0327.2007.00174.x
Bouillon S., Rivera-Monroy V.H., Twilly R.R., and Kairo J.G., 2009, Mangroves, In: Laffoley D.d’A. and Grimsditch G. (eds.), 2009, The management of natural costal carbon sink, IUCN, Gland, Switzerland, pp.53
Bunt J.S., Boto K.G., and Boto G., 1979, A survey method for estimating potential levels of mangrove primary production, Marine Biology, 52: 123-128
https://doi.org/10.1007/BF00390419
Cheeseman J.M., Herendeen L.B., Cheeseman A.T., and Clough B.F., 1997, Photosynthesis and photoprotection in mangroves under field conditions, Plant Cell and Environment, 20: 579–588
https://doi.org/10.1111/j.1365-3040.1997.00096.x
Cintron G., and Schaeffer-Novelli S.Y., 1984, Methods for studying mangrove structure, In: Snedaker S.C.,and Snadaker J. (eds.), The mangrove ecosystem: research methods, UNESCO, Paris, 91-113
Clough B.F., and Sim R.G., 1989, Changes in gas exchange characteristics and water-use efficiency of mangroves in response to salinity and vapor pressure deficit, Oecologia, 79: 38–44
https://doi.org/10.1007/BF00378237
PMid:28312810
Clough B.F., 1992, Primary productivity and growth of mangrove forests, In: Robertson, A.I. and Alongi D.M. (eds.), Tropical mangrove ecosystems, American Geophysical Union, Washington, USA, 225–249
https://doi.org/10.1029/CE041p0225
English S., Wilkinson C., and Basker V., 1997, Survey manual for tropical marine resources (2nd Ed.), Australian Institute of Marine Science, Townsville, 119-195
Forest Department, 2012, Forest cover map Sri Lanka, Forest Department, Sri Lanka
Gammanpila M., Dahanayaka D.D.G.L., and Jayasiri H.B., 2009, Effects of Limnological characteristics on seasonal abundance and distribution of zooplankton of Negombo Lagoon in Sri Lanka, Proceeding of International Conference on Knowledge Management for Sustainable Development, December 2009 in Katmandu, Nepal
HoldrigeL.R., Grenco W.C., Hathy W.H., Liang T., and Tosi J., 1971, Forest environment in tropical life zones: a pilot study, Pregaman Press, New York, 747
Jayakody J.M.A.L., Amrasinghe M.D., Pahalawattarachchi V., and De SilvaK.H.W.L., 2008, Vegetation structure and potential gross primary productivity of mangroves at Kadolkallle in Meegamuwa (Negombo) estuary, Sri Lanka, Sri Lanka Journal of Aquatic Sciences, 13: 95-108
Kathiresan K., and Bingham B.L., 2001, Biology of mangroves and mangrove ecosystems, Advances in Marine Biology, 40: 81-251
https://doi.org/10.1016/S0065-2881(01)40003-4
Kathiresan K., and Khan S.A., 2010, International training course on costal biodiversity in mangroves: course manual, Annamalie University (CAS in Marine Biology, Parangipettai), India, pp.744
Mc Leod E., and Salm R., 2006, Managing mangroves for resilience to climate change, IUCN, Gland, Switzerland
Perera K.A.R.S., Amarasinghe M.D., and Somaratna S., 2013, Vegetation structure and species distribution of mangroves along a soil salinity gradient in a micro tidal estuary on the north-western coast of Sri Lanka, American Journal of Marine Science, 1(1): 7-15
Perera K.A.R.S., Amarasinghe M.D., and Pahalawattaarachchi V., 2010, Effect of vegetation structure on potential gross primary productivity for mangrove ecosystem in Negombo estuary, Sri Lanka, Proceeding of 11th Annual Research Symposium, Faculty of Graduate Studies, University of Kelaniya, Sri Lanka, November 2010 at Kelaniya, Sri Lanka, pp.122
Perera K.A.R.S., and Amarasinghe M.D., 2016, Atmospheric carbon removal capacity of a mangrove ecosystem in a micro-tidal basin estuary in Sri Lanka, Journal of Atmospheric Environment, 134: 121-128
https://doi.org/10.1016/j.atmosenv.2016.03.034
Pinto L., 1986, Mangroves in Sri Lanka, Natural Resource Energy and Sciences Authority of Sri Lanka
Ronnback P., Crona B., and Ingwall I., 2007, The return of ecosystem goods and services in replanted mangrove forests–perspectives from local communities in Gazi Bay, Kenya, Environmental Conservation, 34: 313-324
https://doi.org/10.1017/S0376892907004225
Spalding M.D., Blasco F., and Field C.D., (eds.), 1997, World mangrove atlas, International Society of Mangrove Ecosystems, Okinawa, Japan
Van Zon J.C.J., and Benthem W., (eds), 1994, Conservation management plan, Muthurajawela marsh and Negombo lagoon, Wetland conservation project, Central Environmental Authority, Sri Lanka, Euroconsult, Netherlands
Wijeratne E.M.S., Rydberg L., and Pathirana K.P.P., 2004, Modelling of sea levels, water exchange and dispersion in an intermittently closed tidal estuary: Chilaw lagoon, west coast of Sri Lanka, Proceedings of the 10th Asian Congress of Fluid Mechanics, May 2004, Peradeniya, Sri Lanka
Woodroffe C.D., 1993, Geomorphological and climatic setting and the development of mangrove forests, In: Lieth, H. and Masoom, A. N.. (eds.), Towards the rational use of high salinity tolerant plants, Kluwer Academic Publishers, Netherlands, 13-24
. PDF(593KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. M.A.D. Umayangani
. K.A.R.S. Perera
Related articles
. Mangroves
. Vegetation structure
. Leaf area index
. Gross primary productivity
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)
.png)


