Research Article
Temporal Variations of Micro Benthic Assemblage in the Sangu River Estuary, Bangladesh 
2 Bangladesh Fisheries Research Institute, Saidpur, Nilphamari, Bangladesh
 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2020, Vol. 10, No. 7 doi: 10.5376/ijms.2020.10.0007
Received: 02 Sep., 2020 Accepted: 15 Oct., 2020 Published: 30 Oct., 2020
Begum P., Shamsuzzaman M.M., Mitu S.J., and Ahamed S., 2020, Temporal variations of micro benthic assemblage in the Sangu River Estuary, Bangladesh, International Journal of Marine Science, 10(7): 1-14 (doi: 10.5376/ijms.2020.10.0007)
Temporal distribution of shellfish assemblages, together with water quality data, was conducted in the Sangu river estuary of Bangladesh to assess shellfish's diversity index during winter, pre-monsoon, and monsoon and post-monsoon periods. A total of 15 species of shellfish belonging to 9 families was recorded of which Acetes sp. (25.18%), Matuta victor (18.77%), Exopalaemon styliferus (18.28%), Parapenaeopsis sculptilis (14.22%) were found to be most dominant species during the study period. Significant temporal differences were observed for water temperature, salinity, water transparency, PH and DO. The diversity indices, Shannon-Wiener diversity index and Margalef richness index showed a significant difference among the seasons while no significant difference was observed in the Pielou’s evenness index and Simpson dominance index. The analysis of similarity (ANOSIM) was used to test for significant differences in species assemblages between sampling seasons. At the similarity of 74.8%, three groups were attained while winter and pre-monsoon showed separate clustering from other groups. The Non-metric Multidimensional Scaling (nMDS) showed 50% similarity in all seasons based on Bray-Curtis similarity matrix. The CCA ordination indicated that temperature was the most important environmental parameter shaping the shellfish assemblage structure.
Background
Estuaries are transition zones between sea and fresh water; they are occupied by a combination of freshwater and marine species as well as juveniles (Nabi et al. 2011). The estuarine area plays an important role in terms of both economic and ecological functions for Bangladesh. The area provides important functions including transportation, industry, and tourism and helps to drainage of waste from domestic, industrial and agricultural activities (Heip and Herman, 1995; Raz‐Guzman and Huidobro, 2002). Fishes play an important role in estuaries, as they constitute permanent and temporary community components, and marine species visit these habitats for food, reproduction, growth and protection (Raz‐Guzman and Huidobro, 2002). These estuarine ecosystems offer protection for resident species and a wide range of fresh and marine water species (Cowley and Whitfield, 2002; McLusky and Elliott, 2004; Blaber, 2008). There are 260 species of freshwater finfish, 475 species of marine water fish, and 60 species of shrimp and prawn found in Bangladesh. A total number of 301 species of mollusks and over 50 species of commercially important crustaceans and 76 species of fish from estuaries have been recorded so far in the coastal zone (Quader, 2010). These estuarine areas are highly dynamic and high productive with fish faunas that exhibit abundance and variable composition (Day et al., 1989), therefore, it is necessary to examine the environmental factor that shapes the structure of species assemblages.
The estuarine system of Bangladesh is mainly in the Brahmaputra-Meghana (Gangetic delta), Karnafully, Matamuhuri, Bakkhali and Naaf rivers. Each estuary has its own geographical, hydraulic, sediment logical and biological characteristics. Fish assemblages in estuaries are often variable both in terms of species composition and distribution pattern (Harris et al., 1999). Estuarine fish assemblages often exhibit large year-to-year variations in abundance, species and size composition (Methven et al., 2001). Shellfishes play an important part in estuaries as they constitute permanent and temporary community components, where marine species used these habitats for feeding, reproduction, growth and protection. Various biological and abiotic factors affect the occurrence and habitat of fish and shrimp inside the estuaries. These physico-chemical parameters of Bangladesh's coastal waters are very fluctuating from place to place (Khan, 2005). Thus, the role of seasonal or temporal variability in environmental factors in structuring the shellfish communities in estuaries has been the focus of many studies.
The Sangu River originates in the Arakan Hills of Myanmar and enters Bangladesh near Remarki of Thanchi upazilla of Bandarban district. It is one of the most important estuaries that provides spawning, nursing, feeding ground for numerous fish and shellfish species of the Bay of Bengal and those of upstream. Karnafully River may change environmental parameters that may influence the abundance of fish, shellfish, and other aquatic organism in Sangu River. However, as the shellfish diversity and abundance change temporally and spatially, it is necessary to gather continuous data on the shellfish assemblage and to know their distribution pattern for better management options. However, a notable amount of research has been conducted on different common estuaries of Bangladesh and a very few researches was recorded in the diversity of fish and shrimp of ESBN in the Sangu river estuary (Kamal, 2000; Mamun, 2010; Nabi et al., 2011). Hence, the current study was designed to assess the diversity index of shellfish assemblage in the Sangu river estuary along with their relations with the different environmental parameters.
1 Materials and Methods
1.1 Description of the study area
The Sangu river (locally called shanko) originates in the Arakan Hills of Myanmar and enters Bangladesh near Remarki of Thanchi upazilla of Bandarban district. It flows towards north through Thanchi, Rowangchhari and Bandarban Upazilas of Bandarban district (Islam, 2012). The river's length is 295 km and follows a northerly circuitous course in the Hill Tracts up to Bandarban (Islam, 2012). It is navigable up to 48.27 km from the estuary. The Sangu gas field is located about 50 km southwest of Chittagong and stands at a depth of 10 meters the Bay of Bengal (Banglapedia, 2014). The shellfish samples were collected from the Sangu River's estuary at 22007ʹ N and 91052ʹ E using the estuarine set bag net (ESBN) (Figure 1).
.png) Figure 1 1 Study area map showing the location of the Sangu River |
1.2 Sampling gear
In the Sangu river estuary ESBN locally called ‘behundi jal’ is used for fishing (Figure 2). The set bag net is a fixed, tapering net resembling a trawl net to some extent, set in the tidal stream by attaching it to holdfasts. At least one third of the production of small-scale fisheries of Bangladesh is contributed by ESBN (Shamsuzzaman, 2009). Due to the difficulties in embedding the wooden stakes in the sea bed, this fishing method is restricted to a maximum water depth of about 25 m. The mesh size decreases from 140~20 mm at the mouth to 22~5 mm at the cod end. The length of the net varies from 8.5 m to 41 m, and the height of the mouth opening is 2~7 m and during high tide net submerged under water 1.5~3 m (Islam et al., 1993).
.png) Figure 2 Estuarine set bag net (ESBN) used for sampling |
1.3 Sampling season and time
Shellfish sample was collected during pre-monsoon (April-2014), monsoon (July-2014), post-monsoon (October-2014) and winter (December-2014). Shellfish samples were collected from four different seasons as stated by Mahmood et al. (1992). According to Mahmood et al. (1992), these seasons are the dry winter season, from December to February; the transition period, from March to May (pre-monsoon); the rainy season, from June to September (Monsoon); and the second transition period, between October and November (post-monsoon). Sampling was conducted from ESBN at each sampling period. Shellfish sample was collected every three days before the full moon in each season.
1.4 Sample collection and identification
After collecting the sample from the surveyed area Shellfish were identified up to genus/species level. The shellfish were identified using the species classification proposed by Fischer and Bianchi (1984), Siddiqui and Zafar (2002), Shafi and Quddus (1982), Day et al. (1989), and Sea life base. The total weight and number of each net were also recorded. The following methods determined water quality parameters. Water temperature (Celsius thermometer), Salinity (refractometer) (News-100, TANAKA, Japan), Water pH (pen pH meter) (s327535, HANNA INSTRUMENTS), Transparency (Secchi dise) and Dissolved Oxygen (APHA 1976).
1.5 Data analysis
Diversity of the species assemblage was analyzed by the Shannon-Wiener index (Hʹ), species richness was measured by Margalef index (d) (Margalef, 1968), evenness was measured by Pielou’s index (Jʹ) (Pielou, 1966) and dominance was measured by Simpson index (c) (Shannon and Weaver, 1963; Ramos et al., 2006; Margalef, 1968; Pielou, 1966). The value of Shannon-Wiener diversity index, Margalef richness index, Pielou’s evenness index and Simpson dominance index calculated by using the following formula:
Shannon-Weiner diversity index (H'):
Where, H'=Shannon Wiener index, Pi=ni/N, ni=no. of individuals of a species, N = Total number of individuals
Margalef species richness (d):
d= (S-1)/log (N)
Where, S = Total species, N = Total individuals.
Pielou's evenness index (J'):
J'=H(s)/H(max)
Where, H (s) = the Shannon-Wiener information function and H (max.)=the theoretical maximum value for H(s) if all species in the sample were equally abundant.
Simpson Dominance Index (C):
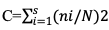
Where, ni = number of individuals in the species; N=total number of individuals and S = total number of species.
A one-way analysis of variance (ANOVA) was used to test for a significant difference in environmental variables (Water parameters), the Shannon-Wiener diversity index (Hʹ), Pielou’s evenness index (Jʹ) and shellfish abundance among seasons. Prior to the ANOVA test, all variables were tested for normality and homogeneity using “Kolmogrouv Smirnov test” and “Levene’s test” (Akin et al., 2005). One-way analysis of Similarity (ANOSIM) was used to conclude the significance of temporal distribution in the structure of shellfish assemblage (Clarke and Warwick, 1994). Similarity percentage analyses (SIMPER) were used to observe each taxon's percentage contribution to the average dissimilarity between the season's combinations (Clarke, 1993). Hierarchical agglomerative clustering with group-averaging linking and non-metric multi-dimensional scaling (nMDS) were performed to investigate similarities among shellfish abundance and analysis was based on the Bray-Curtis similarity measure (Clarke and Warwick, 1994; Bray and Curtis, 1957). For descriptive statistics and ANOVA, test SPSS software V11.5 (Statistical Package for Social Sciences) were used. All the multivariate analyses were performed using the software PRIMER V6 (Plymouth Routines Multivariate Ecological Research) (Clarke, 1993). Canonical correspondence analysis (CCA) was used to investigate the relationship between species assemblage composition and environmental variables. PAST (Paleontological Statistics) version 2.16 was used to perform CCA.
2 Results
2.1 Environmental parameters
Ecological parameters of an aquatic environment mainly temperature, salinity, transparency, dissolved oxygen and pH etc. are varied with the season to season. Water temperature in the studied area varied from 25.47°C to 30.8°C with a mean value of 28.54±2.26°C (Table 1). A significant difference was observed among the seasons (F1,3=27.01;p=0.00).
.png) Table 1 Mean values (± SD with range) of water quality parameters measured in the study area in different seasons |
Salinity values ranged from 7.3 ppt to 25.5 ppt in the Sangu river estuary with a mean value of 17.7±7.68 ppt (Table 1; Figure 3). A significant difference was observed among the seasons (F1,3=33.3;p=0.00). Water transparency varied from 8.3 cm to 22.1 cm in the Sangu river estuary with a mean value of 15.91±6.30 cm (Table 1; Figure 3). A significant difference was found among the seasons(F1,3=11.6;p=0.002). The dissolved oxygen in the study area ranged between 5.10 mg/L to 7.54 mg/L with a mean value of 6.09±1.07 mg/L (Table 1; Figure 3). The difference of dissolved oxygen among seasons was significant(F1,3=24.51;p=0.001). Water pH in the study area varied from 5.7 (in winter and monsoon) to 6.6 (in pre-monsoon) with a mean value of 6.04±0.44 (Table 1; Figure 3). A significant difference was observed among the seasons (F1,3=11.09;p=0.003).
.png) Figure 3 Seasonal variation of water quality parameters in the Sangu river estuary in four seasons |
2.2 Species abundance
From the Sangu river estuary, a total of 1 231 individuals of shellfish were collected during the study. They represented 15 species belonging to 9 families (Table 2). The most dominant family was found to be Penaeidae (3 species) and Palaemonidae (3 species) followed by Portunidae (2 species), Potamidae (2 species). In winter the most dominant species was Acetes sp. (27.83%) followed by Exopalaemon styliferus (15.91%), Matuta victor (15.43%), Parapenaeopsis sculptilis (12.97%), Macrobrachium mirabile (12.10%) and Scylla serrata (5.83%). The most dominant species observed in pre-monsoon was Acetes sp. (29.56%), Parapenaeopsis sculptilis (17.63%), Exopalaemon styliferus (16.30%) followed by Matuta victor (14.36%) and Macrobrachium mirabile (13.41%). In monsoon the most dominant species recorded Matuta victor (16.97%) followed by Exopalaemon styliferus (15.14%), Parapenaeopsis sculptilis (14.08%), Macrobrachium mirabile (12.66%), Potamon sp. (11.41%), Metapenaeus monoceros (9.77%), Scylla serrate (6.02%) and Acanthopotamon sp. (5.54%). Parapenaeopsis sculptilis (19.54%) was the most dominated species in post-monsoon season, while other species contributions were Matuta victor (19.27%), Exopalaemon styliferus (17.52%), Macrobrachium mirabile (11.62%), Scylla serrata (9.85%), Potamon sp. (9.81%) and Penaeus monodon (8.54%). Acetes sp. showed highest species abundance in winter, pre-monsoon and these were 27.83%, 29.56%, respectively, in case of monsoon Matuta victor (16.97%) was dominant, and Parapenaeopsis sculptilis (19.54%) showed high abundance in post- monsoon.
.png) Table 2 Mean values of species abundance in the Sangu river estuary in different seasons |
2.3 Species diversity
2.3.1 Shannon-Wiener Diversity Index (H')
The highest and lowest value of Shannon-Wiener diversity index (H') ranged between 2.31 (in monsoon) and 1.96 (in pre-monsoon) with a mean value of 2.10±0.11 (Table 3; Figure 4). The significant difference was observed among the seasons (F3, 32=3.01, P=0.082) with higher mean diversity value (2.31±0.25) during the monsoon.
.png) Table 3 Mean values (±SD with range) of different diversity index measured in the Sangu river estuary |
.png) Figure 4 Temporal variations of Diversity Index, Species Richness, Evenness Index and Dominance Index at Sangu river estuary in different seasons |
2.3.2 Margalef Richness Index (d)
The minimum Margalef richness index was observed in pre-monsoon (2.55), while the highest value observed in monsoon (3.23) with the mean richness of 2.83±0.43 (Table 3; Figure 4). A significant difference was observed in the mean values of Margalef species richness among seasons (F3.32=3.31;p=0.077).
2.3.3 Pielou’s Evenness Index (J')
Pielou’s evenness index was found the minimum in winter and pre-monsoon (0.94) and maximum value observed in post-monsoon (0.97) with a mean value of 0.95±0.04 (Table 3; Figure 4). No significant difference found among seasons (F3.32=2.64;p=0.21).
2.3.4 Simpson dominance index (c)
The minimum value of Simpson dominance index (c) was observed in pre-monsoon (0.90) and the maximum value was observed in monsoon (0.93) with a mean value of 0.91±0.01 (Table 3; Figure 4). No significant difference found among the seasons(F3.32=1.52; p=0.187).
2.4 Species assemblages
2.4.1 Analysis of Similarity (ANOSIM)
The significant difference was found among the four seasons (Figure 5) and Table 4 represent an analysis of similarity (ANOSIM) where global R was observed 0.836 and P= 0.1%.
.png) Table 4 Results of ANOSIM and SIMPER analysis in the shellfish species abundances between different season’s combinations |
.png) Figure 5 ANOSIM analysis is showing the similarity among the seasons |
2.5 Similarity percentage (SIMPER) between seasons
SIMPER analysis showed the average percent dissimilarity of species between different seasons. 18.60% dissimilarity observed between winter and pre-monsoon, 37.72% dissimilarity observed between winter and monsoon, 42.15% dissimilarity observed between pre-monsoon and monsoon, 37.51% dissimilarity observed between winter and post-monsoon, 39.73% dissimilarity observed between pre-monsoon and post-monsoon, 25.19% dissimilarity observed between monsoon and post-monsoon (Table 5).
.png) Table 5 SIMPER analysis is showing dissimilarity % between different seasons with their contributing shellfish species |
SIMPER analysis showed the average similarity of species in winter season was 80.81%. Acetes sp. (27.83%), Exopalaemon styliferus (15.91%), Matuta victor (15.43%), Parapenaeopsis sculptilis (12.97%), Macrobrachium mirabile (12.10%), Scylla serrata (5.83%) showed the highest similarity in winter; while in pre-monsoon season that average similarity of species was 82.17% and Acetes sp. (29.56%), Parapenaeopsis sculptilis (17.63%), Exopalaemon styliferus (16.30%), Matuta victor (14.36%), Macrobrachium mirabile (13.41%) had shown the highest similarity (Table 6).
.png) Table 6 SIMPER for average similarity and contributing species in each season |
In the monsoon season, the average similarity was observed at 82.89%. Matuta victor (16.97%), Exopalaemon styliferus (15.14%), Parapenaeopsis sculptilis (14.08%), Macrobrachium mirabile (12.66%), Potamon sp. (11.41%), Metapenaeus monoceros (9.77%), Scylla serrate (6.02%), Acanthopotamon sp. (5.54%) showed the highest similarity; while in post-monsoon season that average similarity of species was 85.98% and Parapenaeopsis sculptilis (19.54%), Matuta victor (19.27%), Exopalaemon styliferus (17.52%), Macrobrachium mirabile (11.62%), Scylla serrata (9.85%), Potamon sp. (9.81%), Penaeus monodon (8.54%) had shown the highest similarity (Table 6).
2.6 Cluster analysis
Three marked separations were observed in the abundance of shellfish in different seasons (Figure 6). Three groups were attained at the similarity of 74.8% while winter and pre-monsoon showed separate clustering from other groups.
.png) Figure 6 Cluster analysis based on Bray-Curtis similarity matrix of different seasons |
2.7 Non-metric Multidimensional Scaling (nMDS)
nMDS was performed to investigate similarities among shellfish abundance. Non-metric Multidimensional scaling showed 50% similarity among all the seasons while 60% similarity showed two separate clusters, but 90% similarity observed only between pre monsoon 3 and winter 2 (Figure 7).
.png) Figure 7 Dimensional ordination in nMDS showing the similarity of different seasons |
2.8 Canonical Correspondence Analysis (CCA)
Canonical correspondence analysis showed the relationship between the shellfish assemblage and environmental variables (Figure 8). The CCA ordination indicates that temperature and transparency (best correlated with axis 2) are the two most important environmental parameters shaping the species assemblage structure in the Sangu river estuary than other variables. Here the CCA figure indicates S1=Acetes sp., S2=Matuta victor, S3=Exopalaemon styliferus, S4=Parapenaeopsis sculptilis, S5=Macrobrachium mirabile, S6=Potamon sp., S7=Metapenaeus monoceros, S8=Scylla serrata.
.png) Figure 8 CCA showing the relationship of environmental variables with species assemblage |
3 Discussion
3.1 Water quality parameters
Shellfish distribution within biologically and physically complex estuarine systems influenced by water quality parameters. Environmental parameters such as temperature, salinity, dissolved oxygen, water transparency and pH vary from season to season and play an important role in the abundance and diversity of species, particularly in tropical regions (Blaber, 2008). Fluctuation in water quality parameters influences the estuary's primary productivity, which ultimately affects the fish distribution (Arthington and Welcome, 1995; McAllister et al., 2001). During the present study water temperature was recorded from 25.47°C to 30.8°C in the Sangu River estuary. Das (2005) found the range of water temperature at Bakkhali from 23°C to 30°C. Hasan (2013) recorded the water temperature range at Karnafully river estuary from 25°C to 31°C. Thus, the water temperature in the Sangu river estuary nearly coincided with the reports of mentioned works. Many types of research on fish assemblage in estuaries had shown that salinity plays a major role in shaping assemblage structure (Das, 2005). Islam (2012) recorded the salinity in the Sangu river estuary was ranged from 6.80 ppt to 27.50 ppt. In the present investigation at Sangu river estuary, the salinity ranged between 7.3 ppt to 25.5 ppt which was similar to Islam (2012). He found the salinity in the Karnafully river estuary between 3.30 ppt to 19.93 ppt. Mamun (2004) recorded 9 ppt to 18.5 ppt salinity in the Halishahar coast, Chittagong.
Water transparency in the study area was recorded from 8.3 cm to 22.1 cm. Hoque et al., (1999) recorded transparency in the Matamuhuri River which ranged from 21.5 cm to 77.5 cm. Dissolved oxygen is one of the most important factors for shellfish abundance and distribution. Islam (2012) recorded the Sangu river estuary's DO content as 4.67 ml/L to 8.5 ml/L. In the present investigation, the DO concentration showed a wide range of variation from 5.10 mg/L to 7.54 mg/L that was more or less similar to the above works. The water pH at the Sangu river estuary varied from 5.7 to 6.6. Nabi et al. (2011) observed some variation of pH in Bakkhali estuarine water from 6.3 to 7.7. Boyd (1989) considered standard pH 6 to 9.5. So, the range of pH was in a favorable condition in the study at Sangu river estuary. Das (2005), Mamun (2004), Hossain (2001) also recorded pH which was more or less similar to the present study.
3.2 Species abundance
The present study recorded a total of 15 species of shellfish belonging to 9 families and 3 orders in the Sangu river estuary. Hasan (2013) recorded a total of 18 species of shellfish collected by ESBN from Karnafully river estuary which was more or less similar to the above research works. Islam (2012) was recorded a total of 47 species from ESBN catch at Sangu river estuary among them 16 species were shellfish and the most dominant family in the estuary was Penaeidae followed by Engrualidae and Gobiidae. Hasan (2013) recorded the most dominant family in Karnafully river estuary was Penaeidae and Portunidae reported the most dominant family in the Naaf river estuary was Penaeidae. The result of the present study showed a resemblance to the other findings.
3.3 Species diversity
Seasonal difference in the species diversity is a very common phenomenon in the tropical estuaries, and the presently studied estuary follows the same role. The Shannon-Wiener diversity index (H′) ranged from 1.96 to 2.31 that were more or less similar to the values reported by Islam (2012) in the Sangu river estuary and Hasan (2013) in the Karnafully river estuary where the Shannon-Wiener values were found to be 2.534 to 3.191 and 1.95 to 2.15, respectively. On the contrary, Margalef richness index ranged from 2.55 to 3.23 in the Sangu river estuary. Islam (2012) reported the Margalef richness index of Sangu river estuary was 3.725 to 6.691. The result of the present study was more or less similar with the values reported by Nabi et al. (2011), Kamal (2000) and Hasan (2013), but did not match with Islam (2012) which may be due to the lower number of species available in the present study.
Pielou’s evenness index (J′) was found 0.94 to 0.97 with a mean value of 0.95±0.04 in the Sangu river estuary (Table 3) where Islam (2012) observed Pielou’s evenness index in the Sangu river estuary varied from 0.905 to 0.987. Hasan (2013) found the Pielou’s evenness index in the Karnafully river estuary ranged from 0.93 to 0.96, which coincided with the present findings. The Simpson dominance index was also found to be higher (0.93) at estuarine in the monsoon, and the minimum was in pre-monsoon (0.90) season (Table 3) which indicates that species dominancy was higher in the monsoon season than the other in the study area. Islam (2012) and Hasan (2013) studied at Sangu River and Karnafully River and found that the species dominancy was higher in monsoon which was akin to present findings.
3.4 Species assemblage
The analysis of similarity (ANOSIM) was used to test for significant differences in species assemblages among sampling seasons. In the present study, a significant difference was found among seasons in the analysis of similarity (ANOSIM) where global R was observed at 0.836% and P=0.1%. The most dominant species in the sites were observed among all seasons through SIMPER analysis. Hasan (2013) reported that Exopalaemon styliferus, Parapenaeopsis sculptilis, Charybdis natator and Macrobrachium mirabile were the most dominant species in the Karnafully river estuary. The present study's finding was more or less similar to the above research works done by Hasan (2013). The cluster analysis showed distinct separation among seasons. At the similarity of 74.8%, three groups were attained while winter and pre-monsoon showed separate clustering from other groups. Islam (2012) found three cluster groups at 60% similarity in Sangu river estuary, and Nabi et al. (2011) found two groups at 65% similarity in Bakkhali river estuary. On the other hand, the findings did not match them, which may be due to time and sampling error variation. The Multidimensional Non-metric scaling showed 50% similarity among all seasons while 60% similarity showed two separate clusters, but 90% similarity observed only between pre-monsoon 3 and winter 2. Islam (2012) found 60% similarity in all season in Sangu river estuary, and Nabi et al. (2011) found 65% similarity in all seasons in Bakkhali river estuary for finfish and shellfish. Their findings are dissimilar with the present findings because the study was designed only for shellfish species in the Sangu river estuary.
3.5 Canonical correspondence analysis (CCA)
CCA provided insight into the relationship between the fish assemblage and environmental variables (Marshall and Elliott, 1998). In CCA, species plotted closer to the vector have stronger relationship with them. The CCA of species composition data revealed that temperature was the most significant variables shaping the species composition in the Sangu river estuary. The temperature was strongly correlated with estuarine shellfish communities' distribution as reported by many authors (Islam, 2012; Hasan, 2013). From the CCA ordination plot it is revealed that Matuta victor, Exopalaemon styliferus, Macrobrachium mirabile, Potamon sp., Metapenaeus monoceros has the highest level of temperature preference than the others, while the abundance of Parapenaeopsis sculptilis, Scylla serrata are associated with transparency.
4 Conclusion
As an estuary, the Sangu river estuary is a productive zone. It was found from the present study that seasonal variations occurred not only in total abundance and diversity but also in the structure of the species assemblage of the Sangu river estuary. Acetes sp. was the most dominant shellfish species in the Sangu river estuary contributing 25.18% in total species composition. The temperature was the most important variable shaping the species assemblage in the Sangu river estuary. A moderate, diverse shellfish assemblage was visible there. The findings of this study will serve as fruitful information about shellfish management as well as overall fisheries management of the Sangu river estuary.
Authors’ contributions
This article is based on the first author’s MS studies. PB carried out the fieldwork, analysis, and draft preparation; MMS carried out the supervision, conceptualization, software, analysis, draft preparation, and editing; SJM participated in its design and coordination and helped draft and edit the manuscript. SA read and revised the manuscript. All authors read and approved the final manuscript.
APHA, 1976, Standard Method for the examination of waste water, American Public Health Association, Washington, D.C. 1193
Akin S., Buhan E., Winemiller K.O., and Yilmaz H., 2005, Fish assemblage structure of Koycegiz Lagoon-Estuary, Turkey: Spatial and temporal distribution patterns in relation to environmental variation, Estuarine, Coastal and Shelf Science, 64(4): 671-684
https://doi.org/10.1016/j.ecss.2005.03.019
Arthington A.H., and Welcome R.L., 1995, The conditions of large river systems of the world, In: Armantrout NB, editor. Conditions of the World’s Aquatic Habitats, Proceedings of the World Fisheries Congress, Theme 1, Lebanon, New Hampshire, USA, Science Public Inc, 45-75
Banglapedia, 2014, Sangu river estuary, Bangladesh, Retrieved from http://en.banglapedia.org/index.php?title=Bangladesh [Accessed date 2nd Dec 2014].
Blaber S.J., 2008, Tropical estuarine fishes: ecology, exploitation and conservation. John Wiley & Sons
https://doi.org/10.2307/1942268
Boyd C.E., 1989, Water quality management and aeration in shrimp farming, Printed from fisheries and allied aquaculture department, Series No. 2. Allied Aquaculture Experimental Station, Auburn University, Alabama, February, 1989
Bray J.R., and Curtis J.T., 1957, An ordination of the upland forest communities of southern Wisconsin, Ecology Monographs, 27: 325-349
Clarke K.R., 1993, Non‐parametric multivariate analyses of changes in community structure, Australian J. of Ecology, 18(1): 117-143
https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Clarke K.R., and Warwick R.M., 2001, Change in marine communities: An approach to statistical analysis and interpretation, Natural Environment Research Council of the United Kingdom, 144
Cowley P.D., and Whitfield A.K., 2002, Biomass and production estimates of a fish community in a small South African estuary, Journal of Fish Biology, 61: 74-89
https://doi.org/10.1111/j.1095-8649.2002.tb01763.x
Das N.G., 2005. Livelihood and Resource Assessment for Aquaculture Development in Waterlogged Paddy lands: Remote Sensing, GIS and Participatory Appraisal, A joint Application of GOB-DANIDA and IMS, CU, 122
Day Jr. J.W., Hall C.A., Kemp W.M., and Yanez-Arancibia A., 1989, Estuarine ecology, John Wiley & Sons, New York, 576
Fischer W., and Bianchi G., 1984, FAO species identification sheets for fishery purposes, Western Indian Ocean (Fishing Area 51), Vol. 1-5
Hasan A., 2013, Temporal distribution of shellfish assemblage in the Karnafully river estuary, Bangladesh, M. Sc. Thesis, Faculty of Fisheries, Sylhet Agricultural University, Sylhet-3100
Harris S.A., and Cyrus D.P., 1995, Occurrence of fish larvae in the St Lucia Estuary, KwaZulu-Natal, South Africa, South African Journal of Marine Science, 16(1): 333-350
https://doi.org/10.2989/025776195784156601
Heip C.H.R., and Herman P.M.J., 1995, Major biological processes in European tidal estuaries: a synthesis of the JEEP-92 Project, Hydrobiologia 311: 1-7
https://doi.org/10.1007/978-94-009-0117-9_1
Hoque S.M.A., Zafar M., and Mahmood N., 1999, Temporal and spatial variation of phytoplankton with emphasis on Skeletonema costatum in the Matamuhuri river estuary (chakaria mangrove ecosystem), Bangladesh, Pakistan Journal of Marine Sciences, 8(1): 29-39
Hossain M.S., 2001, Biological aspects of the coastal and marine environment of Bangladesh, Ocean & Coastal Management, 44(3-4): 261-282
https://doi.org/10.1016/S0964-5691(01)00049-7
Islam M.S., Khan M.G., Quayum S.A, Sada M.N., Chowdhury Z.A., and Huq Q.M., 1993, Studies on the interactive marine fisheries of Bangladesh, Working Paper 89, Bay of Bengal Program, Madras, India
Islam J., 2012, Seasonal variation of fish assemblage in Sangu river estuary, Chittagong, Bangladesh, M.S. Thesis, Institute of Marine Sciences and Fisheries, University of Chittagong, Bangladesh
Kamal A.H.M., and Khan M.A.A., 2009, Coastal and estuarine resources of Bangladesh: management and conservation issues, Maejo International Journal of Science and Technology, 3(2): 313-342
Kamal M.M., 2000, Temporal and spatial variation in species diversity of fishes in the Karnafully River Estuary, M. Sc. Thesis, Institute of Marine Sciences and Fisheries, University of Chittagong
Khan H.M.M., 2005, Temporal and spatial distribution of Ichthyofauna in the karnaphully River-estuary with special emphasis on the physico-chemical parameters, Chittagong Institute of Marine Sciences and Fisheries, Chittagong University, Chittagong, pp.99
Mahmood N., Chowdhury M.J.U., Haider S.M.B., and Chowdhury S.R., 1992, A review of the state or environment relating to marine fisheries of Bangladesh, IMSF, University of Chittagong, Bangladesh, pp.66
Mamun M.A., 2004, Water quality consideration for sustainable aquaculture development in the Halishahar coast, Chittagong, M. Sc. Thesis, IMSF, University of Chittagong, pp.66
Mamun M.A., 2010, A comparative study on finfish and shellfish assemblage structure between Bakkhali and Matamuhuri river estuaries, M.Sc. Thesis, Institute of Marine Sciences and Fisheries, University of Chittagong
Margalef R., 1968, Perspectives in Ecological Theory Chicago Illinois University, Chicago Press
Marshall S., and Elliott M., 1998, Environmental influences on the fish assemblage of the Humber estuary, UK, Estuarine, Coastal and Shelf Science, 46(2): 175-184
https://doi.org/10.1006/ecss.1997.0268
McLusky D.S., and Elliott M., 2004, The Estuarine Ecosystem: Ecology, Threats and Management, Third ed. Oxford University Press, 214
https://doi.org/10.1093/acprof:oso/9780198525080.001.0001
McAllister D.E., John F., Davidson N., Delany S., and Seddon M., 2001, Biodiversity Impacts of Large Dams Background Paper No. 1 prepared for IUCN/UNEP/WCD International Union for Conservation of Nature and Natural Resources and the United Nations Environmental Programme, pp.49
Methven D.A., Haedrich R.L., and Rose G.A., 2001, The fish assemblage of a Newfoundland estuary: diel, monthly and annual variation Estuarine, Coastal and Shelf Science, 52(6): 669-687
https://doi.org/10.1006/ecss.2001.0768
Nabi M.R., Mamun M.A., Ullah M.H., and Mustafa M.G., 2011, Temporal and spatial distribution of fish and shrimp assemblage in the Bakkhali river estuary of Bangladesh in relation to some water quality parameters, Marine Biology Research, 7(5): 436-452
https://doi.org/10.1080/17451000.2010.527988
Pielou E.C., 1966, The Measurement of Diversity in Different types of Biological collections, Journal of Theoretical Biology, 13: 131-144
https://doi.org/10.1016/0022-5193(66)90013-0
Quader O., 2010, Coastal and marine biodiversity of Bangladesh (Bay of Bengal), in proceeding of International Conference on Environmental Aspects of Bangladesh (ICEAB10), Japan, 83-86
Ramos S., Cowen R.K., Ré P., and Bordalo A.A., 2006, Temporal and spatial distributions of larval fish assemblages in the Lima estuary (Portugal), Estuarine, Coastal and Shelf Science, 66(1-2): 303-314
https://doi.org/10.1016/j.ecss.2005.09.012
Raz‐Guzman A., and Huidobro L., 2002, Fish communities in two environmentally different estuarine systems, Mexico Journal of Fish Biology, 61: 182-195
https://doi.org/10.1111/j.1095-8649.2002.tb01770.x
Shamsuzzaman M.M., 2009, Temporal and spatial assemblages of fish fauna around the Sandwip Island with special reference to some physico-chemical parameters, M.Sc. Thesis, Institute of Marine Sciences and Fisheries, University of Chittagong
Shannon C.E., and Weaver W., 1963, The mathematical theory of communications, University of Illinois Press, Urbana, II, 125
Shafi M., and Quddus M.M.A., 1982, Fisheries Resources of Bangladesh (Bangladesher Matshaya Sampad, in Bengali), Bangla Academy, Dhaka, Bangladesh
Siddiqui M.Z.H., and Zafar M., 2002, Crabs in the Chakaria Sundarban area of Bangladesh, The Journal of Noami, 19(2): 61-77
. PDF(3249KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Begum Prianka
. Md. Mostafa Shamsuzzaman
. Sabrina Jannat Mitu
. Saokat Ahamed
Related articles
. Acetes sp.
. Fish Assemblage
. Parapenaeopsis Sculptilis
. Sangu river estuary
. Species richness
Tools
. Email to a friend
. Post a comment


