Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2013, Vol. 3, No. 4 doi: 10.5376/ijms.2013.03.0004
Received: 02 Dec., 2012 Accepted: 11 Jan., 2013 Published: 11 Jan., 2013
Latypov, 2013, Barrier and Platform Reefs of the Vietnamese Coast of the South China Sea, International Journal of Marine Science, Vol.3, No.4 23-32 (doi: 10.5376/ijms.2013.03.0004)
The composition and spatial distribution of the coral communities of the barrier reefs of Giang Bo, Ly Son island and the platform reef at Bach Long Vi Island were described in detail for the first time for Vietnamese waters. In common, more 260 species of corals and their accompanying species of macrobenthos were found. Acroporids, poritids, and mussids among the scleractinian corals dominated. Monospecific aggregations of Alcyonarian Sinularia and Lobophytum and the hydroid Millepora were rather numerous. Based on its geomorphological characteristics, coral species diversity and zonal distribution, the reefs of entire area are comparable with ribbon and platform reefs on the Great Barrier Reef in Australia and to the barrier reefs of the Philippines and Indian Ocean.
1 Results and Discussion
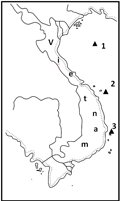
The south–north transects (Figure 1). The bottom area adjoining to the reef base at a depth of 17 m was a flat platform with silt–sand sediments with debris of invertebrate skeletons. The depth gradually decreased by 1.5~2.0 m from the 40th to the 100th meter of the transect. Massive and sporadic encrusting colonies of scleractinian occurred. Colonies of Heliopora occurred most often, Pachyseris, Mycedium, Pavona, Montipora, Podobacia, Goniopora, Alveopora, Lobophyllum, Pectinia, and Euphyllia were rather common, Galaxea, Herpolitha, Fungia, Polyphyllia, and large colonies Porites were sometimes recorded, and Acropora and small of branching Porites occurred sporadically. The coral covering of the substrate was 7–10%. The associated macrofauna presented by singular individuals of the bivalve mollusks Atrina vexillum and Malleus malleus and of the sea stars Linckia laevigata and Culcita novaeguineae. The latter two species also recorded in the same numbers in all other zones of the reef. At the distance of 100–160 m from the beginning of the transect, the percentage of coral covering of substrate pronouncedly increased, from 10 up to 72%, with the same thing occurring to the size of colonies and species diversity of scleractinian Acropora most of all.
|
|
The buttress system began from the 160th meter of the transect (Figure 2). The species diversity of the corals sharply increased (by 1.5 times) concurrently with the variety of their growth forms. The coral covering of substrate increased to 100%. Young settlements of scleractinian rather often occurred on an old lamellar colony of Acropora of a 100×60 cm area (Table 1).
.png)
Figure 2 Structure of corals settlements at buttress system on the reef Giang Bo (depth 12 m) |
.png)
Table 1 Settlements of small coral on dead lamellar Acropora |
This zone extended almost to 70 m. The following approximately 40 m of the transect were a zone of Acropora, in which monospecies settlements of A. cytherea, A. hyacinthus, A. formosa, and Porites rus with individual colonies of Goniastrea, Astreopora, Pocillopora, Montipora, Heliopora, and massive forms of Porites, were clearly distinguished.
At a depth of 3~5 m, at the 240th~250th meter of transect, the reef flat zone extending by 200~300 m began with individual channels. It characterized by a continuous covering of substrate by corals (Figure 3) and interrupted by hollows oriented from northeast to southwest. The ample tangled settlements, up to 100~150 m wide, were composed of A. cytherea, A. hyacinthus, A. formosa, P. rus, Montipora aequituberculata, the seaweed Chnoospora implexa and sea mat Zoanthus sp. Individual colonies of scleractinian and small bioherms spread in channels of sand sediments. The holothurian Holothuria atra (0.2 ind./m2), the bivalves Tridacna crocea (up to 2 ind./m2), and T. squamosa (up to 0.1 ind./m2) were the most abundant of the associated fauna, including the gastropods Cymatium spp. and Oliva spp. (up to 3 ind./m2) occurring in the sand.
|
|
Corals were associated with bivalves, with domination of Arca ventricosa (10~15 ind./m2) and Beguina semiorbiculata (up to 10 ind./m2), common Septifer bilocularis, Pinctada margaritifera and Cardita variegata, as well as the gastropods Tectus niloticus, Trochus maculates and Turbo petholatus (Table 2). The projective cover of substrate by corals varied from 10% up to 100% and included more than 100 species in its composition.
|
|
From the 670th to the 673rd meter of the transect, in a rather limited bottom area at a depth of 9~11 m, large colonies of A. formosa and A. florida up to 2.5 m in diameter with clumps of fungiid in between were replaced by settlements of A. formosa with infundibular M. aequituberculata and lamellar–ramose Merulina ampliata reaching 1.5 m in diameter. The percentage of coral covering of the substrate sharply decreased with 680th meter of the transect, and the species composition of corals was reduced by almost 2 times. The coral settlements looked like individual patches of several colonies. Rather small bioherms sometimes occurred. By the 700th meter of the transect at a depth of about 9 m only individual scleractinian or bunches of 3~5 colonies, whose projective cover of the bottom was about 3%~7% occurred.
Transect from the southwest to the northeast side of the reef (Figure 4). At a depth of 25 m, on the fore reef platform with slightly silted sand sediments with detached boulders and spalls of dead coral, isolated colonies of alcyonarian, gorgonarians and scleractinian occurred. A gradual decrease in depth observed to the northeast for 200 m of transects. The number of coral colonies significantly increased along with an increase in their size. Sinularia dura, Sarcophyton sp., Porites lobata, Isopora palifera, Pocillopora verrucosa, and Montipora venosa were the most common corals. The coral covering of the substrate did not exceed 5%~7%. At a depth of 12 m, bioherms from 0.9 up to 2.5 m high and up to 1.5~4 m in diameter were common. They consisted of polyspecies settlements of alcyonarian and scleractinian, in which Sinularia, Porites, Favia and Favites prevailed. The first dominated by substrate cover, up to 30%, the rest by number species up to 7~9. The scleractinian of the Euphyllidae, Meruliniidae, and Mussidae families (by 2~3 species) and individual colonies of Acropora, Montipora, Astreopora, Pocillopora, and Seriatopora occurred in that part of the reef rather often. Sponges, bivalves, gastropods and the sea urchin were characteristic in the associated invertebrate fauna for the area (see Table 2). Shrubs of the macrophytes Asparagopsis taxiformis, Padina australis, Caulerpa racemosa, Amphiroa fragilissima, and Halimeda sp. The composition of the fauna of reef slopes also had high degree of similarity, 78.9% of the species were in common. The slightly lesser similarity of the reef slope communities explained by the greater richness of macrophytes at the reef of Ly Son island.
|
|
The outer reef slope. This characterized by the presence of two morphologically differing parts, the lower and upper (Figure 5, nomenclature by Picard, 1967; Battistini et al., 1975). In the lower part (of the reef slope platform, at depths from 30 to 10 m) there were numerous alcyonarian and ahermatypic corals Balanophyllia, Dendrophyllia, and Tubastrea and hermatypic scleractinian were presented by stony and encrusting forms, Pachyseris, Leptoseris, Pectinia, Echinophyllia, Euphyllia, and Mycedium. The percentage of substrate covering by corals in that part of slope was rather low, 0.1%~5%. The upper part of the reef slope (buttress system, at a depth from 10 to 3 m) characterized by the presence of a system of channels, niches and spurs. Coral species diversity and substrate covering by corals (up to 40%) was higher there. Patches of monospecies settlements of the alcyonarian Sinularia or Lobophytum, of the scleractinian Acropora and Porites, and of Millepora and Heliopora occurred. In the lower areas of the upper part of the reef slope massive and encrusting forms of colonies of Goniopora, Goniastrea, Favia, Favites, Platygyra, Echinophyllia, Turbinaria, and Montipora prevailed. They replaced higher up by branching forms of colonies, primarily Acropora, and species that are able to inhabit an environment of intense hydrodynamics, viz., Acropora digitifera, A. humilis, Pocillopora verrucosa, Goniastrea retiformis, Millepora platyphylla, and Heliopora coerulea often occurred.
|
|
Reef flat. Normally this was a vast zone with a continuous substrate cover of corals interrupted by sandy channels. This zone characterized by the development of dominant species of Acropora and Montipora and of branching forms of Porites (A. humilis, A. monticulosa, M. aequituberculata, M. porites, P. cylindrica, etc.). The significant role in the formation of the reef flat community belonged to the alga Chnoospora, Turbinaria, Asparagopsis, Hyphnea, Amphizoa, and Peissonelia. The projective cover of substrate by corals was 75%~100% as a rule.
|
|
1.3 Reef of Bach Long Vi Island
At the beginning of transect, on the northeastern part of the reef, at a depth of 16 m is a fore reef platform or a fore reef. This is an area of slightly silty coarse and medium-grained sand with inclusions of blocks and fragments of dead coral. Sparse colonies of alcyonarian, gorgonians, and scleractinian encountered here. A gentle slope was observed stretching 250–300 m northwest. With decreasing depth, the number of large colonies of corals markedly increased. Soft corals Sinularia dura, Lobophytum sp., Sarcophyton sp., scleractinian Favites abdita, Isopora palifera, Montipora venosa, Porites lobata, and Goniopora stokesi predominated in terms of frequency. The coral covering of substrate was 5%~7%. The reef slope down to about 12 m depth covered with coral thickets and large bioherms up to 4~5 m high (Figure 6). This reef zone dominated by two to three species of Sinularia, Lobophytum, and Sarcophyton (Figure 7), massive colonies of Porites, plate-like and encrusting Montipora, Pectinia, Pachyseris, plate-like and digitate Acropora, and branching Pocillopora. The macrophytes Turbinaria, Asparagopsis and Gracilaria also encountered. The covering of substrate was up to 30%~40% for corals and 10%~20% for algae. Large colonies of Porites provided the basis for bioherms (Figure 8). Among other common inhabitants of the reef were the starfishes A. planci, C. novaeguineae, and L. laevigata, the sea urchin D. setosum, and mollusks P. margaritifera, Pteria penguin, Tridacna squamosa, Lambis lambis and Conus textile. With decreasing depth to 8~10 m, the number of corals increased and hence so did the coral cover up to 7%~10%. The most widespread on this part of the reef slope were S. dura, Acropora humilis, M. venosa, P. lobata, G. stokesi, Platygyra lamellina, Leptoria phrygya, Lobophyllia hemprichii, Merulina ampliata and the hydroid coral Millepora platyphylla (coral cover 40%~60%). Individual bushes of macrophytes P. australis, Turbinaria sp. and A. taxiformis, the sponge P. testudinaria, the starfish L. laevigata, the sea urchin E. diadema, and the holothurian H. atra were encountered.
|
|
|
|
At a depth of 2~6 m, the upper reef slope with a well-defined system of spurs and grooves (buttress system) supports the highest species diversity of scleractinian (more than 150 species). A community of Acropora and Montipora occurred this depth. Acropora cytherea, A. hyacinthus, A. formosa, A. grandis, Montipora hispida, M. aequituberculata, M. vietnamensis, Pocillopora verrucosa and Seriatopora hystrix were dominant. As a rule, large colonies of Acropora millepora, A. specifera and M. hispida (or M. eaequituberculata) formed stacked aggregations that covered 100% of the substrate over several tens of square meters. In this zone, the calcareous algae Poroliton sp. and Halimeda sp., the alcyonarian Cladiella pachyclados, the scleractinian Acropora robusta, A. humilis, A. monticulosa, I. palifera, Pocillopora woodjonessi, S. hystrix and Galaxea astreata, various poritids and faviids (5~7 species of each), the hydroid M. platyphylla, macrophytes Turbinaria, Gracilaria and Asparagopsis were widespread. Along with them, the starfishes L. laevigata, C. novaeguineae, the sea urchins D. setosum, E. diadema, the holothurians H. atra, and the individual mollusks T. squamosa, Trochus maculatus, Cyprea arabica, and C. textile encountered.
|
|
|
|
|
|
Along the 100~200 meter's using frames, divided into 100 squares 10 cm2, assessed the number of species branch, massive, incrusting and funnelform colonies of scleractinian and the degree of coverage of the substrate of corals and algae. Number mass a species of mollusks and echinoderms counted up on the area of 5~10 m2. The number of colonies of corals recorded at transect, is calculated using the Shannon diversity index using the formula H' =ï¼Σ pi log Pi, where Pi is the prevalence of colonies giving genera (Bakus, 1990). The wide use of the underwater photo technique was a specific feature of this research. The population density of associated macrofauna was assessed from the projective area of the bottom (corals) taken for the count area. The reefs characterized based on cumulative data, not considering annual variations.
Acknowledgement
. PDF(556KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Yuri Latypov
Related articles
. Coral reef
. Species composition
. Structure
. Vietnam
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)
.png)
.png)
.png)