The A.O. Kovalevsky Institute of Biology of the Southern Seas, National Academy of Sciences of Ukraine, 2, Nakhimov av., Sevastopol 99011, Crimea, Ukraine

Author

Correspondence author
International Journal of Marine Science, 2013, Vol. 3, No. 15 doi: 10.5376/ijms.2013.03.0015
Received: 28 Feb., 2013 Accepted: 29 Mar., 2013 Published: 01 Apr., 2013
Stelmakh, 2013, Microzooplankton Grazing Impact on Phytoplankton Blooms in the Coastal Seawater of the Southern Crimea (Black Sea), International Journal of Marine Science, Vol.3, No.15 121-127 (doi: 10.5376/ijms.2013.03.0015)
Introduction
Microzooplankton is generally considered as heterotrophic and mixotrophic organisms able to switch to phagotrophy, with individual linear dimensions lesser than 200 µm (Calbet and Landry, 2004; Calbet, 2008). Protozoa and early life stages of mesozooplankton as well as larval stages of benthic animals are all microzooplankton. Protozoa are represented by flagellates, including dinoflagellates, ciliates and small foraminifers (Sherr and Sherr, 2007; Calbet, 2008). Measurements performed in seawater areas of all known types point out that, on the average, heterotrophic flagellates contribute to the total microzooplankton biomass as large as 64% (Sherr and Sherr, 2009). Early developmental stages of mesozooplankton are mainly nauplii of copepods (Calbet, 2008). Until recently, the investigations conducted over the Black Sea have only assessed the biomass produced by individual groups of microzooplankton (Zaika and Averina, 1968; Pavlovskaya, 1976; Polikarpov et al., 2003) setting aside the associated functional aspects, in particular the rates of microzooplankton grazing on phytoplankton. Research in this field began in 2005, during the international expedition to the western Black Sea on board of the R/V “Vladimir Parshin” (Stelmakh et al., 2009). These results confirm that microzooplankton is the major consumer of phytoplankton in the World Ocean. On the average, 70% of the primary production in the open sea and about 60% in the coastal zone are removed by microzooplankton (Calbet, 2008). Some authors (Sherr and Sherr, 2007) interpret the predatory pressure of protozoa, primarily heterotrophic dinoflagellates, on diatoms, as an effective tool regulating bloom formation. Knowing that annual primary production in the Black Sea is mainly due to diatoms (Stelmakh et al., 1998), clarifying the role that microzooplankton plays in formation of diatom blooms appears the task of high priority.
For gaining insight into this aspect, we studied seasonal dynamics of microzooplankton predatory pressure on the phytoplankton blooms which emerged near the shores of Sevastopol and village Katsiveli, the latter situated eastward on the Crimean coast of the Black Sea.
1 Results
1.1 The seasonal dynamics of the specific rate of microzooplankton grazing
In 2006~2007, the observations related to microzooplankton predatory pressure on phytoplankton in the Sevastopol bay (st.1) evidenced that over a year the estimates could differ nearly 8 times as large (Figure 1).
|
.png)
Figure 1 Seasonal dynamics of the microzooplankton grazing (g) on phytoplankton (1), the ratio of grazing to phytoplankton growth rate (g/µ), (2) and phytoplankton biomass (3) in the surface waters of the Sevastopol bay (A), Quarantine bay (B) and in the open coastal waters near Kruglaya bay (C) in 2006~2007
|
Maximums (1.6~1.7 d-1) were registered in June 2006, when an intensive bloom of Chaetoceros spp. was fading away, and in May 2007, prior to another outbreak of these diatoms. Minimums (0.2~0.3 d-1) were measured from October 2006 till February 2007. The records from st.3 located in the open coastal sea water next to the Kruglaya bay (Figure 1C) show similar seasonal variation. In the Quarantine bay (st.2), the microzooplankton grazing activity intensified to the peaks ranged between 1.4~1.6 d-1 in June 2006 and in May 2007. Noteworthy, it was only slighter lesser (1.1~1.5 d-1) during November~December 2006 (Figure 1B). The facts that phytoplankton biomass in the Quarantine bay has been 2~2.5 times as large as in the Sevastopol bay and at the Kruglaya bay region and that Chaetoceros spp., the favorite prey item for the microzooplankton, dominated in the rich phytoplankton production can account for these late autumn~early winter maximums.
In summer 2010, the sea water was 4℃ warmer than usual; according to the records, in the phytoplankton biomass near the shores of Sevastopol and village Katsiveli dinoflagellates prevailed for most time of the year. In the samples collected near Sevastopol the small-celled
Skeletonema costatum, the abundance of which were as large as to conform to a bloom level (2~3 × 10
6 cells/L), dominated only in February. In March, the predatory pressure of microzooplankton increased to 0.5 d–1 in the Sevastopol bay and to 0.7 d
-1 in the open coastal water area neighboring the Kruglaya bay (
Figure 2). In May, at the intensive growth period of
Emiliania huxleyi (2.2×10
6~2.5×10
6 cells/L) and at low total phytoplankton biomass (30~70 mg C/m
3), the specific microzooplankton grazing rate decreased to 0.2 d
-1 in the Sevastopol bay and to 0 d
-1 near Kruglaya bay; from June to August it was again increasing in these two locations to 0.6~0.8 and 0.8~1.1 d
-1, correspondingly. Simultaneously, the portion of small forms of mixotrophic dinoflagellates, namely
Prorocentrum cordatum and
P. micans, responsible for larger values of summer phytoplankton biomass has increased (70% of the total biomass) in the microplankton. In the Quarantine bay, over the observation period the phytoplankton loss due to microzooplankton grazing inconsiderably fluctuated between 0.4~0.7 d
-1.
|
.png)
Figure 2 Seasonal dynamics of the microzooplankton grazing on phytoplankton (1), the ratio of grazing to phytoplankton growth rate (2), and phytoplankton biomass (3) in the surface waters of the Sevastopol bay (A), Quarantine bay (B) and in the open coastal waters near Kruglaya bay (C) in 2010
|
In 2010, lowest estimates of phytoplankton biomass and microzooplankton grazing impact were registered near the shore of village Katsiveli. During the year, the microzooplankton predatory pressure varied in the narrow range of 0~0.5 d-1 (Figure 3) reducing to minimal in September at st.4, and in August~ September at st.5, that coincided with the minimal total phytoplankton biomass (20~30 mgС/m3), i.e., with the local prey deficiency. Maximums were registered in November at st.4 and in May at st.5.
|
.png)
Figure 3 Seasonal dynamics of the microzooplankton grazing on phytoplankton (1), the ratio of grazing to phytoplankton growth rate (2), and phytoplankton biomass (3) in the surface waters near Katsiveli in 2010: A – St. 4, B – St. 5
|
It is generally accepted that water temperature influences both phytoplankton growth and grazing activity of zooplankton. Therefore, all data on the microzooplankton grazing impact that has been generated for 2006~2007 and 2010 was divided into two sets. One dataset comprised the cold-season (November~April) estimates obtained when the average temperature of the sea was 11℃ (± 2℃), and the other represented the warm season (May~October) when the seawater temperature for all stations and for years was averaged 23℃ (± 4℃). Analysis of the data has shown that in 2006 and 2007, the specific rate of microzooplankton grazing on phytoplankton during the warm season made 1.19 d-1 on the average for all sampling stations, being practically twice as large as that during the cold season (Table 1). In the warm season of 2010, it was estimated 0.52 d-1 that did not reliably differ from the cold season. The shortage of prey (low phytoplankton biomass) in the warm months of 2010 and, probably, the changes in the phytoplankton taxonomic structure (the dominance of dinoflagellates) have decreased the predatory control by microzooplankton. Then, logarithmic dependence between the specific rate of phytoplankton consumption by microzooplankton and chlorophyll-а concentration in the plankton was determined (Figure 4).
|
.png)
Table 1 The microzooplankton grazin of phytoplankton (g), the concentration of chlorophyll-a (Chl a), specific diatom biomass (Bdiat.) and dinophyta (Bdinof.) algae in the studied coastal waters of the Black Sea near Sevastopol and Katsiveli
|
|
.png)
Figure 4 Dependence of the microzooplankton grazing from chlorophyll-a concentration in the coastal waters of the Black Sea near Sevastopol and Katsiveli in the warm period in 2010
|
Obviously, microzooplankton grazing rate increases with growing content of chlorophyll-a. The curve describing microzooplankton grazing rate shows the fastest increase in the beginning, within the range of chlorophyll-a concentration from 0.1 mg/m3 to 0.4 mg/m3. The content of this pigment growing on, the microzooplankton grazing pressure slowed down; its minimum was associated with the concentration of chlorophyll-a close to 0.6 mg/m3. At the significance level of p=0.05, the specific microzooplankton grazing rate described by 66% of available prey abundance. This equation and the plot were generated using software Sigma Plot 2001 for Windows (Version 7.101). Important factor is the quality of the prey, particularly the volume of microalgal cells. Equation derived using multiple regression method indicates that the variability of the specific grazing activity of microzooplankton by as much as 71% depends on chlorophyll-а content in the microplankton and on the average volume of phytoplankton cells:
g = 0.321•chl а – 0.061•Vcell + 1.260
(R2 = 0.71, SE = ± 0.20, p = 0.05)
where g is the specific microzooplankton grazing rate, d–1; chl а–chlorophyll-а concentration, mg/m3, and Vcell – the average volume of phytoplankton cells, µm3.
1.2 The seasonal dynamics of phytoplankton biomass and the primary production loss due to microzooplankton
The records made for 2006~2007 evidence three peaks of phytoplankton biomass which formed in the Sevastopol bay over the year (
Figure 1). Two first, in June and in September 2006, were both generated by blooming
Chaetoceros spp. and estimated close to 150 and 330 mg С/m
3, correspondingly. The third, in February 2007, was due to the small form of
S. costatum, with the largest estimate of 60 mg С/m
3. Next time the microalgal biomass increased to a maximum of 250 mg С/m
3 in June 2007. At the periods of biomass maximums in the bay, the ratio between the phytoplankton loss and growth (g/µ) was never greater than 50%~80%, being in conformity with the relative percentage of primary production consumed a day by the microzooplankton and explaining the repeated increases of phytoplankton production to highest. In July and December 2006 and in March 2007, the plankton biomass decreased to lowest whereas the ratio stayed as high as 100%~160%. It means that the quantity of organic substance of the phytoplankton consumed by the microzooplankton was equal to or greater than the primary production, therefore the low phytoplankton biomass. In Quarantine bay, at phytoplankton biomass maximums g/µ ratio was estimated 55%~80%. In November 2006 and in March 2007, the biomass estimates dropped to lowest values (10~20 mg С/m
3) but g/µ ratio was as high as 120%~220%. These months the daily loss of phytoplankton biomass due to microzooplankton grazing was larger than the daily primary production, therefore the low biomass estimates. In the sea area off the Kruglaya bay’s shore maximums of phytoplankton biomass, ranging between 80 and 170 mg С/m
3, were recorded in June 2006 and 2007 and in October 2006. In these periods 125%~175% of the daily phytoplankton production was removed by the microzooplankton grazing. Interestingly, that in the beginning of blooms in September 2006 and in May 2007, when phytoplankton biomass was rising after a minimum and the total abundance
Skeletonema costatum and
Chaetoceros socialis was 2~3×10
6 cells/L, the phytoplankton mortality due to microzooplankton predation did not exceed 50%~55%.
Compared to 2006~2007, over most part of 2010 the phytoplankton biomass was noticeably less. In the Sevastopol bay, the largest estimates in 150 and 300 mg C/m
3, were registered in February and in July, correspondingly (
Figure 2). In February, the dominant taxa were the diatom
S. costatum and in July, two small dinoflagellates,
Gymnodinium simplex and
P. cordatum, accounted up to 87% of the total phytoplankton biomass. The loss of primary production due to microzooplankton grazing during these two periods was assessed 33% and 76%, correspondingly. In February, June and September the microalgal biomass in the Quarantine bay was estimated as high as 120 mg C/m
3, and the g/µ ratio was 50%~60%. In July, in the near-shore area adjoining the Kruglaya bay, phytoplankton biomass increased maximally to 100 mg C/m
3; simultaneously, the g/µ ratio was 80%. According to the year-round observations from two sampling stations near village Katsiveli, the portion of phytoplankton consumed by microzooplankton, or g/µ ratio, was mainly below 100% (
Figure 3). Frequent upwelling – downwelling events induced by wind prevent accumulation of phytoplankton biomass in this region of the sea (
Subbotin et al., 2007).
Based on the records for 2006~2007 and 2010, in the examined locations of the Black Sea the specific rate of phytoplankton mortality due to microzooplankton grazing was evaluated, on the average, as 59% of the primary production for the warm season and 70% for the cold season, making the average of 65% for the complete observation period.
At the periods of phytoplankton biomass maximums the g/µ ratio mainly varied from 33% to 80% but sometimes it was estimated zero; therefore the average of 50%.
2 Discussion
The study conducted in the coastal seawater areas of the Black Sea has shown that seasonal variability of the specific grazing rate of the microzooplankton, absolute values of which were comparable to the estimates of the specific growth rate of the phytoplankton, varied very broadly (
Stelmakh et al., 2009). The amount and quality of prey phytoplankton have been the main factors which regulated the microzooplankton predatory pressure on phytoplankton. Quality, or prey selectivity, has been the principal factor where the abundance of prey phytoplankton approximated a saturation point. For instance, in October 2006, when phytoplankton biomass in the Sevastopol bay was as large as 350 mg С/m
3, the microzooplankton grazing impact was negligible. The phytoplankton biomass then was predominantly due to two large-celled species, the diatom
Pseudosolenia calcar-avis and the dinoflagellate
Ceratium furca. As a prey item, the former is not in favor with the microzooplankton (
Stelmakh et al., 2009), and the latter episodically provides a prey to only a few heterotrophic dinoflagellates, e.g.,
Protoperidinium steinii (
Olseng et al., 2002). It was reported earlier (
Stelmakh et al., 2009) that in September~October 2005, during autumn diatom blooms in the western Black Sea, as the portion of
Pseudosolenia calcar-avis increased in the total phytoplankton biomass, the specific rate of phytoplankton mortality due to microzooplankton grazing gradually decreased.
Emiliania huxleyi is also a prey of minor interest to microzooplankton; in October~November 2010, the observations related to the autumn bloom of this species of coccolithophorids in the western Black Sea have shown that in the localities where
E. huxleyi had the abundance as high as 1.5~3 million cell/L the consumption of phytoplankton by microzooplankton often ceased (
Stelmakh and Babich, 2011).
The phytoplankton consumed by the microzooplankton varies from long-chain diatoms (
Sherr and Sherr, 2007) to large dinoflagellates (
Olseng et al., 2002). According to our observations, the mortality of phytoplankton due to microzooplankton grazing was usually largest during the periods when diatoms of
Chaetoceros spp. dominated in the plankton, therefore a deduction about a favorite prey item for the microzooplankton. Some proofs to this can be found elsewhere (
Shinada et al., 2000).
During abnormally warm year 2010, the biomass of phytoplankton at the studied locations was relatively low. Generally, the largest contribution was due to dinoflagellates with specific growth rate less in 2~3 times in comparison with diatoms (
Stelmakh et al., 2010). The average phytoplankton loss due to microzooplankton grazing during warm months of 2010 was significantly (nearly two times) lower compared to warm seasons of 2006 and 2007. Presumably, the scarcity of prey and the half reduced diatom portion in the phytoplankton assemblage could account for the decline in the specific rate of microzooplankton impact.
As soon as phytoplankton growth rates exceeded the rate of mortality due to microzooplankton grazing, phytoplankton bloom started. As our observations indicate, difference between the two rates was largest at the peak of blooming. Near Sevastopol, phytoplankton blooms emerged when g/µ ratio was estimated between 33% and 80%, i.e., 50% on the average.
At the studied areas of the Black Sea the primary production grazed by microzooplankton varied widely over the year, yielding the annual average of 65%. This means that microzooplankton removes most of the yearly primary production in the region, leaving to mesozooplankton the lesser portion of microalgal production. For comparison, in nutrient-rich coastal waters of the World Ocean is 10% on the average (
Calbet and Landry, 2008).
3 Conclusion
In the studied areas at Sevastopol and Katsiveli (southern Crimea, Black Sea), the specific rate of microzooplankton grazing depended on the quantity and the quality of available phytoplankton; Chaetoceros spp. and Skeletonema costatum have been the favorite preys. Phytoplankton blooms in the region were usually caused by diatoms. Blooming started when the average ratio between the rates of phytoplankton loss due to microzooplankton predation and phytoplankton growth reached approximately 50%. It was estimated that the annual consumption of phytoplankton by the microzooplankton in the sampling areas reached 65%. The recent observations prove the crucial role of microzooplankton in the matter and energy transfer from phytoplankton to higher trophic levels in the coastal Black Sea.
4 Data and Methods
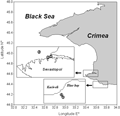
During 2006~2007 and 2010, 86 daily surveys were made at the examined locations. Three sampling stations were situated near of the coast of Sevastopol and two were located near village Katsiveli, southern Crimea’s shore (
Figure 5). Station 1 (44°37.25′N, 33°30.43′E) was located in the Sevastopol bay at a distance of 50 m from the shore; st. 2 (44°36.50′N, 33°29.53′E) in the Quarantine bay, 30 m off the shore; st. 3 (44º37.20′N, 33º29.50′E) was positioned near the Kruglaya bay at a distance of 500 m from the shore. Stations 4 and 5 (44°39.18′N, 33°97.93′E and 44°39.04′N, 33°98.03′E, correspondingly) were situated in the Blue bay, 200~250 m from the coast. Except for st.3 where the depth was 30 m, the depth at other stations varied between 15~18 m.
The rates of phytoplankton growth and loss as result of microzooplankton grazing were determined using dilution procedure (
Landry and Hasset, 1982). The major advantage of this method is that it assesses the rate of total phytoplankton growth along with the rate of microzooplankton grazing on the phytoplankton. Samples of seawater (12~15 L) were taken from the sea surface (~ 0.5 m depth) early in the morning using Niskin bottle.
The design of daily experiments and the pertinent computations have been published early (
Stelmakh et al., 2009). Chlorophyll-
a concentration was measured using fluorimetric technique (
Protocols JGOFS, 1994). Samples (1 L) were filtered onto Whatman GF/F filters. After filtration filters were placed in 90% acetone (5 mL) and chlorophyll was extracted for 24 h at 4℃ in the dark. Before and after acidification fluorescence was measured on a fluorometer (excitation 440 to 480 nm, emission > 665 nm), which was calibrated with pure chlorophyll-
a (Sigma Chemical Co). The precision of these measurements was high, with a relative standard deviation of 5%.
For determination of phytoplankton biomass and species composition, 3~4 L samples of sea water were condensed under the nucleopore membranes (1 µm pore size; the product of the Institute of Nuclear Researches, Dubna, Russia) in the inverse filtering plexiglass funnel, (
Sorokin et al., 1975). Samples after reducing of volume were fixed with neutralized 1%-formaldehyde (final concentration in the sample) and immediately processed. The numbers and dimensions of microalgae were measured in a 0.1 mL drop in 3~5 replications under the light microscope ZEISS Primo Star (×400). The precision of these measurements was with a relative standard deviation of 25%. Unfortunately the abundance and biomass of microzooplankton was not determined. Mathematical treatment of all data involved using Microsoft Office Excel 2007 and Sigma Plot 2001 software for Windows.
Acknowledgements
My special thanks to Irina I. Babich (IBSS), for her valuable help in measuring phytoplankton biomass and phytoplankton species identification.
Calbet A, and Landry M.R., 2004, Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems, Limnol Oceanogr., 40: 51-57
http://dx.doi.org/10.4319/lo.2004.49.1.0051
Calbet A., 2008, The trophic roles of microzooplankton in marine systems, ICES J. Mar. Sci., 65: 325-331
http://dx.doi.org/10.1093/icesjms/fsn013
JGOFS Protocols, 1994, Protocols for the Joint Global Ocean Flux Study (JGOFS) Core Measurements, Manual and Guides, 29, UNESCO
Lаndry M.R., and Hassett R.P., 1982, Estimating the grazing impact of marine micro-zooplankton, Marine Biology, 67: 283-288
Olseng C.D, Naustvoll L.J, and Paasche E., 2002, Grazing by the heterotrophic dinoflagellates Protoperidinium steinii on a Ceratium bloom, Mar. Ecol. Prog. Ser., 225:161-167
http://dx.doi.org/10.3354/meps225161
Pavlovskaya T.V., 1976, Distribution of microzooplankton in the coastal seawater of the Black Sea, Marine Biology, 36: 75-83
Polikarpov I.G., Saburova M.A, Manzhos L.A, Pavlovskaya T.V, and Gavrilova N.A., 2003, Biological diversity of microplankton in coastal zone of the Black Sea near Sevastopol (2001 - 2003) . In: The current state of coastal waters of Crimea (the Black Sea sector), Sevastopol (in Russian)
Sherr E.B., and Sherr B.F., 2007, Heterotrophic dinoflagellates: a significant component of microzooplankton biomass and major grazers of diatoms in the sea, Mar. Ecol. Prog. Ser., 352: 187-197
http://dx.doi.org/10.3354/meps07161
Sherr E.B., and Sherr B.F., 2009, Capacity of herbivorous protists to control initiation and development of mass phytoplankton blooms, Aquat. Microb. Ecol., 57: 253-262
http://dx.doi.org/10.3354/ame01358
Shinada A., Ikeda T., Ban S., and Tsuda A., 2000, Seasonal changes in micro-zooplankton grazing on phytoplankton assemblages in the Oyashio region, western subarctic Pacific, Plankton Biol. Ecol., 47: 85-92
Sorokin Yu.I., Sukhanova I.N., and Konovalova G.V., 1975, The primary production and the phytoplankton of the equatorial divergence area in the eastern Pacific Ocean, Proc. Institute of Oceanology, Ac. Sci. USSR, 102: 108-122 (in Russian)
Stelmakh L.V., Babich I.I., Tugrul S., Moncheva S., and Stefanova K., 2009, Phytoplankton Growth Rate and Zooplankton Grazing in the Western Part of the Black Sea in the Autumn Period, Oceanology, 49: 83-92
http://dx.doi.org/10.1134/S000143700901010X
Stelmakh L.V., and Babich I.I., 2011, Factors controlling the autumn Emiliania huxleyi bloom in the western Black Sea, In: Black Sea outlook: 3rd Biannual Scientific Conference and UP-GRADE BS-SCENE Project Joint Conference (1-4 November 2011, Odessa, Ukraine)
Stelmakh L.V., Yunev O.A., Finenko Z.Z., Vedernikov V.I., Bologa A., and Churilova T.Ya., 1998, Peculiarities of seasonal variability of primary production in the Black Sea, In: Ecosystem modeling as a management tool for the Black Sea. Dordrecht: Kluwer Academic Publishers, l: 93-104
Stelmakh L.V, Kuftarkova E.A, and Babich I.I., 2009, Seasonal variation of the phytoplankton growth rate in coastal waters of the Black Sea (near Sevastopol), Sea Ecol. Journal., 5: 74-87 (in Russian)
Stelmakh L.V., A.I Akimov, E.A. Kuftarkova, I.I. Babich, and Kozhemyaka A.B., 2010, Using a variable fluorescence in vivo to assess the functional status of phytoplankton, Environmental Systems Control, Sevastopol, 13: 263-268 (in Russian)
Subbotin A.A., Troshchenko O.A., Lomakin P.D., and Shchurov S.V., 2007, Main features of the hydrological structure and the seawater dynamics on the Crimean shelf and their influence on the near-shore mariculture farming, In: Mariculture of mussels on the Black Sea, Sevastopol: EKOSI-Gidrofizika (in Russian)
Zaika V.E, and Averina, T. J., 1968, The abundance of ciliates in the plankton of the Sevastopol bay of the Black Sea, Oceanology, 8: 1071-1073 (in Russian)

 Author
Author  Correspondence author
Correspondence author
.png)
.png)
.png)
.png)
.png)