Marine sponges are a rich source of biologically active secondary metabolites with novel chemical structures. Most bioactive compounds from sponges can be classified as anti-inflammatory, antitumor, immunosuppressive or neurosuppressive, antiviral, antimalarial, antibiotic and antifouling agents. The chemical diversity of sponge products is remarkable. In addition to the unusual nucleosides, bioactive terpenes, sterols, cyclic peptides, alkaloids, fatty acids, peroxides, and amino acid derivatives (which are frequently halogenated) have been described from sponges (Joseph and Sujatha, 2011; Sipkema et al., 2005).
The immune system has evolved through millions of years to combat the invading pathogens. Without this highly sophisticated defense system, human beings and other animals would succumb in an environment infested with hostile, invisible microbes and other infectious agents. The immune system of the body not only recognizes and fights against exogenous intruders but also sometimes destroy abnormal endogenous cancer cells. The functioning of such a system is of great significance for the survival of any animal which is affected by new entry chemicals. As a result, there is a suppression of immune response in the animals and the animals are susceptible to microbial invasion. Hence it is imperative to protect the organisms that are very useful to human beings and also human from pathogenic organisms. In this concern many chemical drugs are developed to enhance immunity but all these drugs leave side effects so a search for immunomodulating drug from natural products got a global attention. To utilize the bioactive compounds for modulating human immune system, experimental studies are needed by using some mammalian experimental system(Kalirajan et al., 2013).
In the present study the eighteenth fraction of column separated extracts of Aurora globostellata 3-hydroxytetradecanoic acidwas fraction to be the major constituents. So this fraction was used for Immuno- modulatory studies.
Immunostimulants and Rodents
Rodents or murine immune system is an experimental system to explore the bioactive potential of any natural or synthetic drug so as to extrapolate the results to human immune system. In order to screen the natural products for immunomodulating activities, a number of studies had been carried out using rodent models. The aqueous suspension of dried latex of Calotrophis procera showed anti-inflammatory property in rat models (Ranjit et al., 2008). The water extract of Azadirachta indica exerted significant anti-inflammatory activity in the cotton pellet granuloma assay in rats (Ray et al., 1996).
Gupta et al (2006) has highlighted the therapeutic potential of immunomodulatory agents from plant products. Galina et al (2009) had listed out several plants that were proved to be immunomodulators (Kannan et al., 2009). The extracts of the plant Rubia cardifolia have been tested successfully for immunomodulatory activities. (Like plant bioactive material several immunomodulative bioactive compounds had been isolated from marine natural products.
The bioactive compound Latrunculin A isolated from Latrunculia magnifica was found to inhibit prostrate tumor cell invasion and hypoxia inducible factor-1 activation of breast tumor cells (El Sayed et al., 2008). The Spongistatin compound was isolated from Spongia sp. induced apoptosis in leukemia cells including those that over express the protein. The sponge Suberea mollis was found to contain subereaphenol A that show antioxidant activity (Abou-shoer et al., 2008). Acanthomine A isolated from Acanthostrongylophora sp. was reported to be cytotoxic to mammalian cancer cell (Ibrahim et al., 2008).
Likewise many new compounds are isolated from marine sponges to stimulate or suppress immunity. As the availability of marine sponge derived bioactive compound is meager, chemical synthesis of the bioactive compounds are carried out to develop possible drugs for immunomodulation. Hence an attempt has been made to identify few bioactive compounds in the spongeA. globostellata (Ag)to evaluate the immunity modifying activity using clinical trials in rodent model.
2 Materials and Methods
2.1 Sponge and extract preparation
Sponge Aurora globostrellata (7.85 kg each) were collected from Rameswaram (9°28′N, 79°12′E) coastal area and washed with sea water, air dried and chopped into small size before being ground into fine paste. The sponge was successively extracted with solvents with an increasing polarity (Ethyl acetate). The sponge extracted material was used for further analysis.
2.2 Immunity study in murine system
2.2.1Animals and treatment
For the experimental study, Wister rats were chosen. The rats were obtained from Madras Medical College, Chennai and reared in laboratory under standard conditions of light and darkness (12-12 h) and temperature (22±2℃). The rats were fed Standard laboratory mice pellet feed (Lipton Ltd., Mumbai) (Consisting protein 15~17%, fat 4~5%, carbohydrate 45~55%, fiber 15%, vitamin A 7000 IU/kg, vitamin E 40 mg/kg, vitamin K 2mg/kg, vitamin B l mg/kg Hawk-Oser salt 11 mg/kg) and water ad libitum to all the animals. The access to animal room was limited and kept to minimum.
For the experimental study rats weighing 150 to 210 gm (30 days old) were recruited from the acclimatized stock. The rats were grouped into several groups and each group with six individuals. These animals were housed in a specially designed (polyethylene cage) cage with provision for systematic supply of pellets and water. The animals were trained to take water from a feeding bottle kept in cage.
Sponge Aurora globostrella extract and standard drug treatment was given to animals for 3 to 5 weeks. During treatment pellet feed and water were given in ad libitum. Food consumption, general condition and other symptoms were observed daily and body weights were recorded weekly. For treatment with sponge purified compound, the doses for the treatment were fixed three groups. In the present study the bioactive compounds were separated using column chromatography. The sixteenth fractions separated from the column showed many bioactive compounds. The dominant peak showed the presence of 3-hydroxytetradecanoic acid. From these compound fractions three different concentrations were prepared using Alsevier's solution. Three different concentrations were 50 mg/kg, 150 mg/ kgand 250 mg/kg.
2.2.2 Immunization
The test animals were divided into equal groups for stimulation with sheep red blood cells (SRBC). For stimulation with antigen, the rats were immunized (i.p.) with SRBC suspended in normal saline (0.15 M). Approximately 25×106 cells/mLwere administered for the primary and 50×106 cells/mLfor the secondary immunization two weeks after the primary dose. Unstimulated mice were treated similarly except immunization with SRBC antigen. The stimulated rats were treated with different doses of sponge extract .For each concentration of sponge extract and control groups, triplicate experiments were maintained.
2.2.3Experimental system
Blood samples of stimulated rats were collected on the first and second weeks following sponge extract treated by cardiac puncture, after anesthetizing the rats with chloroform. The serum was separated for each group separately and kept at -20℃ till analyzed. Sodium citrate (2.8 g/100mL) was used in collecting whole blood and leukocyte rich plasma for lymphocyte subset numeration.
Immunological assays
a) Humoral immune response
I. Antibody titre, II. B cell E rosette assay: to enumerate B lymphocytes.
b) Cell-mediated immune response (CMI)
I. Delayed type hypersensitivity (DTH), II. T cell E -rosette assay: to estimate of T lymphocytes.
2.2.4 Haemagglutination antibody titre (HA) (SRBC-Antigen)
The rats were divided into five groups consisting of six animals each Rats in group I (control group) received vehicle only for 14 days. Groups II received standard drug Dexamethasone (0.5 mg/kg). On 4th and 11th day as a single dose. Rats in treatment with group III were given Immun Aid tab. (immunostimulatory drug) daily for 12 days. Group IV, V and VI were given sponge extract (50 mg/kg, 150 mg/kg, 250 mg/kg). On 4th and 11th day as a single dosing respectively. On 3th and 8th day of study, rats from all the groups (i.e. group I to V were immunized and challenged respectively, with SRBCs in normal saline (0.1 mL of 20% SRBCs) intraperitonially. Blood was withdrawn on 7th and 14th day from heart puncture method, by using chloroform to give mild anesthesia to all rats group. The obtained blood was centrifuged to raise serum, normal saline was used as a diluents and SRBCs count was adjusted to (0.1 mL of 20% SRBCs). Each well of a microtitre plate was filled initially with 25±l of saline and 25±l of serum was mixed in the first well of micro titre plate. Subsequently the 25 ±l diluted serum was removed from first well and added to the next well to get twofold dilutions of the antibodies present in the serum. Further twofold dilutions of this diluted serum were similarly carried out till the last well of the first row (11th well), so that the antibody concentration of any of the dilutions is half of the previous dilution. 20±l SRBC (0.1% of SRBCs) were added to each of these dilutions and the plates were incubated at 37℃ for one hour and then observed for haemoagglutination (Agarwal et al., 1999; Dhasarathan et al., 2010). The highest dilution giving haemagglutination was taken as the antibody titre. The antibody titres were expressed in the graded manner, the minimum dilution (1/2) being ranked as 1, and mean ranks of different groups were compared for statistical significance.
2.2.5 B cell rosette assay
Methodology
Blood is collected from sponge extract treated, control and standard drug treated rat as mentioned in earlier. B cell count in the blood was carried out by the following method.
Five to ten ml of blood was collected and it was introduced into sterile conical flask/beaker containing (4-5) sterile glass beads. It was then continuously swirled until sounds were heard from the beads. This indicates that all the fibrins have adhered to the beads. This blood was considered as defibrinated blood. This defibrinated blood was taken and diluted with equal volume of physiological saline. 3 mL of the lymph prep solution was taken in a centrifuge tube. The tube was kept in slanting position and 9 mL of diluted blood was slowly added along the sides of the centrifuge tube using Pasteur pipette. Care was taken so that the FICON layer of the lymphoprep solution present in the centrifuge tube was not disturbed. The content of the centrifuge tube was then centrifuged at 1600 rpm for 20 min. The interphase (containing lymphocytes) was removed using pipette. The cells were washed with 1 mL saline and excess FICON was removed. The sample was again washed with 1 mL of saline after centrifugation the supernatant was decanted by inverting the tube over a filter paper after all saline was drained; the pellet was then resuspended in 300 µL of RPMI 1640 medium.
Twelve to fourteen centimetre of drinking straw was cut. One end of the straw was slantly cut and sealed by slightly heating the tip in a flame. Nylon wool fibres were finely teased using a pair of forceps and the teased fibres were packed (loosely) into the straw. Adding 5 mL of physiological saline washed the packed nylon wool column. A small opening was made at the sealed end of the straw to drain the physiological saline. After washing with physiological saline, the nylon wool was then filled with 3 mL of RPMI 1640 medium in a horizontal position. The nylon wool column was kept in the incubator (at 37℃ for 30 min) in horizontal position. This process activates the nylon wool column.
Resuspended lymphocytes were loaded into the activated nylon wool column then the column was held vertically above an eppendrof tube, now hot saline (about 60℃) was slowly dripped into the column. The hot saline passing out of the column was collected in the eppendrof tube, which contain and T lymphocytes. After hot saline elution, cold saline in then dripped was through the column. The column in gently squeezed to release the adhered B cells (repeat twice). The cold saline dripping out of the column was collected in another eppendrof tube. 0.2 mL of the saline containing B lymphocyte (from the eppendrof tube containing B cell) was taken in a separate eppendrof tube. To this 0.2 mL of 1% SRBC was added and then the mixture was centrifuged for 12 minutes at 1600 rpm. After centrifugation the sample were incubated in an icebox or refrigerator (at 4℃) for 5 minutes. After cold incubation, the pellet in the eppendrof tube was resuspended by gentle flushing with a Pasteur pipette. Then a drop of it was taken in a clean dry slide, observed and enumerated B cells under the microscope (20×/40×) for rosettes. Numbers of B cell rosettes formed were observed among hundred lymphocytes observed/mL was tabulated.
2.2.6 Delayed type hypersensitivity (DTH) response in rats
On 14th day of the study, all the groups I to IV were immunized with SRBCs (0.1mL of 20% SRBC i.p.) in normal saline. On day 21st all animals from all the groups were challenged with 0.03 mL of 20% SRBCs in sub plantar region of right hind paw. Foot pad edema in rat was used for detection of cellular immune response. On 21st day, injection of 0.1 mL of 20% SRBCs in the sub plantar region of right hind paw in the volume of 0.03 mL and normal saline in left hind paw in same volume as well as the purified compound (50 mg/kg, 150 mg/kg and 250 mg/kg) doses was given. Foot pad reaction was assessed every 3, 6, 9, 12 and 24 hrs. On 22nd day, in terms of increase in the thickness of footpad as a result of hypersensitivity reaction due to edema, the thickness of the right hind footpad was measured using vernier caliper. The footpad reaction was expressed as the difference in the thickness (mm) between the right foot pad injected with SRBC and the left footpad injected with normal saline.
2.2.7 T cell rosette assay
Methodology
Blood is collected from sponge extract treated, control and standard drug treated rat as mentioned in earlier section. T cell count in the blood is carried out by the following method.
Five to ten ml of blood was collected and it was introduced into sterile conical flask/beaker containing (4~5) sterile glass beads. It was then continuously swirled until no sounds were heard from the beads. This indicates that all the fibrins have adhered to the beads. This blood was considered as defibrinated blood. This defibrinated blood was taken and diluted with equal volume of physiological saline. 3mL of the lymphoprep solution was taken in a centrifuge tube. The tube was kept in slanting position and 9 mL of diluted blood was slowly added along the sides of the centrifuge tube using Pasteur pipette. Care was taken so that the FICON layer of the lymphoprep solution present in the centrifuge tube was not disturbed. The content of the centrifuge tube was then centrifuged at 1600 rpm for 20 min. The interphase (containing lymphocytes) was removed using pipette. The cells were washed with 1mL saline and excess FICON was removed. The sample was again washed with 1 mL of saline after centrifugation the supernatant was decanted by inverting the tube over a filter paper after all saline was drained; the pellet was then resuspended in 300 µL of RPMI 1640 medium.
Twelve to fourteen centimetre of drinking straw was cut. One end of the straw was slantly cut and sealed by slightly heating the tip in a flame. Nylon wool fibers were finely teased using a pair of forceps and the teased fibers were packed (loosely) into the straw. Adding 5 mL of physiological saline washed the packed nylon wool column. A small opening was made at the sealed end of the straw to drain the physiological saline. After washing with physiological saline, the nylon wool was then filled with 3 mL of RPMI 1640 medium in a horizontal position. The nylon wool column was kept in the incubator (at 37℃ for 30 minutes) in horizontal position. This process activates the nylon wool column.
Resuspended lymphocytes were loaded into the activated nylon wool column. Then the column was held vertically above an eppendrof tube, now hot saline (about 60℃) was slowly dripped into the column. The hot saline passing out of the column was collected in the eppendrof tube, which contain and T lymphocytes. 0.2 mL of the saline containing T lymphocyte (from the eppendrof tube containing T cell) was taken in a separate eppendrof tube. To this 0.2 mL of 1% SRBC was added and then the mixture was centrifuged for 12 minutes at 1600 rpm. After centrifugation the sample were incubated in an icebox or refrigerator (at 4℃) for 5 minutes. After cold incubation, the pellet in the eppendrof tube was resuspended by gentle flushing with a Pasteur pipette. Then a drop of it was taken in a clean dry slide, observed and enumerated T cells under the microscope (20×/40×) for rosettes. Number of T cell rosettes formed were observed among hundred lymphocytes observed was tabulated.
3 Results and Discussion
3.1 Immunity Study in Rats
The effect of the ethyl acetate extracts of Aurora globostellata was tested for their immumunomodulatory effect in murine models. To test the efficacy of the extracts of A. globostellata, three different does viz; 50 mg/kg, 150mg/kg, 250 mg/kg were used. For immunological study both cell medicated and antibody mediated responses were evaluated. To evaluate the efficacy of the extract to modulate cell mediated immune (CMI) response, Delayed Type of Hypersensitivity (DTH) reaction was studied. For evaluating Antibody mediated immune (AMI) response Haemoagglutination titer (HA titer) was estimated in control and sponge extract treated rats.
3.2 Antibody titre
The extracts of Aurora globostellata (Ag), (50 mg/kg, 150 mg/kg, and 250 mg/kg) produced a dose related increase in the primary and secondary antibody formation with the maximum increase of 120.12±45.6 and 415±202.2 at a dose of 250 mg/kg after 7th and 14th days respectively. The result indicates that 3-hydroxytetradecanoic acid in the extracts of Ag elevated primary and secondary antibody production significantly (P<0.01). The effect was more in secondary immune response (Table 1).
.png)
Table 1 Effect of the extract of A. globostellata on Haemoagglutination (HA titre) assay in Rat challenged with antigen
|
3.3 Delayed-type hypersensitivity response (DTH)
The extracts of Aurora globostellata (50-250 mg/kg) increased DTH in Rats (Table 2, Figure 1).
.png)
Table 2 Effect of the extract of Aurora globostellata on SRBC induced delayed type hypersensitivity (DTH) in Rat
|
.png)
Figure 1 Effect of the extract of S.inconstans var.globosa on SRBC induced delayedtype hypersensitivity (DTH) in Rat
|
The DTH response, which is a direct correlation of cell mediated immunity (CMI), was found to be significantly increased on treatment with sponge extract. During CMI responses, During CMI responses, sensitized T-lymphocytes, when challenged by the antigen, are converted to lymphoblast’s and secrete lymphokines attracting more scavenger cells to the site of reaction. The in filtering cells are thus immobilized to promote defensive (inflammatory) reaction. Increase in the DTH response indicates the stimulated effect on lymphocyte and accessory cell type required for the expression of reaction (Solanki et al., 2010; Dhasarathan et al., 2010).
3.4 Effect of the extracts of S. inconstans on T- cell and B-cell count:
The effect of three concentrations of the extract of A. globostellata was tested in Rat to find out whether this extract can influence the lymphopoeiosis (T-cell and B-cell). In the control rat the amount of B-cell was 20±1% but in standard drug given rat, it was 26±1% on 7th day 57.6±1 on 14th day. In immunosuppressed, Dexamethazone treated rats it was 14±1 on 7th day and 15±1 on 14th day. In A. globostellata extract treated rat, B-cell count was increased to 34±1 on 7th day and 23±0.6 on 14th day at 250mg/kg treated rat. Like B-cell the total number of T-cell count also increased due to the treatment with extract. When compared to B-cells, T-cells count was high (Table 3). The extracts of Ag were found to enhance T-cell and B-cell production after their production was suppressed by immune suppressive drug.
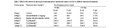
Table 3 Effect of the extract of Aurora globostellata andon lymphocyte count in the Rat in different duration of treatment
|
The results of the present study indicated that the extracts of Aurora globostellata have bioactive compounds which can stimulate both cell mediated and humoral immune response. The hike in T-cell and B-cell population in Rats treated with the extract showed that the extract has metabolites to enhance lymphopoiesis in Rat. As Jensen et al (1994) reported, immune enhancing properties of Ag could be useful in the adjuvant treatment of various diseases. Hence a further research is in progress to find out the exact compound that enhances T-cell and B-cell immunity.
4 Conclusion
The extracts of sponge were tested to find out whether it can do any role in immunomodulation. Both cell mediated immunity and antibody mediated immunity were analyzed using murine model after administering the sponge extracts. The extracts of sponge were found to enhance antibody titre and B-cell count. Total T-cells and Delayed type hypersensitive reactions were also elevated after the administration of sponge extract. From the study it is quite evident that the extract of the sponges are good in immunomodulation.
Acknowledgement
Authors are thankful to Dr. A.J.A.Ranjit Singh, Principal, Sri Paramakalyani College, Alwarkurichi, Tirunelveli, Tamilnadu, Lab facilities and encouragement. We thankful for supporting agency of Department of Science and Technology, Govt. of India, New Delhi.
Agarwal R., Diwanay S., Patki P., and Patwardhan B., 1999, Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extracts in experimental immune inflammation. Journal of Ethnopharmacology, 67: 27-35
http://dx.doi.org/10.1016/S0378-8741(99)00065-3
Chairman K., Ranjit Singh A.J.A., and Ramesh M., 2012, Screening Twelve Species of Sponges for Biomedical Activity in Gulf of Mannar Tuticorin Coast, International Journal of Marine Science, 2: 43-50
Dhasarathan P., Gomathi R., Theriappan P., and Paulsi S., 2010, Immunomodulatory activity of alcoholic extract of different fruits in mice. Jour. of Appl. Sci. Res. 6(8): 1056-1059
Gupta R.S., Kachhawa J.B.S., and Sharma R., 2006, Antispermatogenic effect of Nyctanthus arbortristis in male albino rats. Pharmacology online, 2: 261-273
Joseph B., and Sujatha S., 2011, Pharmacologically Important Natural products from Marine Sponges. Journal of Natural Products, 4: 5-12
Kalirajan A., Karpakavalli M., Narayanan K.R., Ambiganandham K., Ranjitsingh A.J.A., Sudhakar S., 2013, Isolation, characterization and phylogeny of sponge - associated bacteria with antimicrobial and immunomodulatory potential, Int. J. Curr. Microbiol. App. Sci., 2(4): 136-151
Kannan M., and Singh R., 2009, Phytochemistry and immunopharmacological investigation of Rubia cordifolia Linn (Rubiaceae), Pharmacology online, 3: 653-662
Ranjit M.S., Singh R., and Gokulshankar S., 2008, Enhanced phagocytocsis and antibody production by Tinospora cardifolia-A new dimension in immunomodulation. Afr. J. of Biotech. 7(2): 81-85
Ray A., Banerjee B.D., and Sen P., 1996, Modulation of humoral and cell-mediated immune responses by Azadirachta indica in mice, Indian J. Exp. Biol, 34: 698-701
Sipkema D., Franssen Maurice C.R, Osinga R, and Tramper J., 2005, Marine Sponges as Pharmacy, Marine Biotechnology, 7: 142-162
Solanki Y.B, Sunita M., and Jain, 2010, Immunomodulatory activity of Ayurvedic plant Aparajita (Clitoria Ternattea L) in male Albino Rats, Glo. Jour. of Sci. Fron. Res., 10: 2-8
 Author
Author  Correspondence author
Correspondence author
.png)


